Striatal spine plasticity in Parkinson's disease: pathological or not?
- PMID: 20082980
- PMCID: PMC3076277
- DOI: 10.1016/S1353-8020(09)70805-3
Striatal spine plasticity in Parkinson's disease: pathological or not?
Abstract
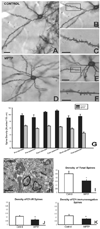
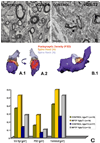
Parkinson's disease (PD) is characterized by a dramatic loss of dopamine that underlies complex structural and functional changes in striatal projection neurons. A key alteration that has been reported in various rodent models and PD patients is a significant reduction in striatal dendritic spine density. Our recent findings indicate that striatal spine loss is also a prominent feature of parkinsonism in MPTP-treated monkeys. In these animals, striatal spine plasticity is tightly linked with the degree of striatal dopamine denervation. It affects predominantly the sensorimotor striatal territory (i.e. the post-commissural putamen) and targets both direct and indirect striatofugal neurons. However, electron microscopic 3D reconstruction studies demonstrate that the remaining spines in the dopamine-denervated striatum of parkinsonian monkeys undergo major morphological and ultrastructural changes characteristic of increased synaptic efficacy. Although both corticostriatal and thalamostriatal glutamatergic afferents display such plastic changes, the ultrastructural features of pre- and post-synaptic elements at these synapses are consistent with a higher strength of corticostriatal synapses over thalamic inputs in both normal and pathological conditions. Thus, striatal projection neurons and their glutamatergic afferents are endowed with a high degree of structural and functional plasticity. In parkinsonism, the striatal dopamine denervation induces major spine loss on medium spiny neurons and generates a significant remodeling of corticostriatal and thalamostriatal glutamatergic synapses, consistent with increased synaptic transmission. Future studies are needed to further characterize the mechanisms underlying striatal spine plasticity, and determine if it represents a pathological feature or compensatory process of PD.
Conflict of interest statement
The authors certify that there is no conflict of interest related to the content of this publication.
Figures
Similar articles
-
Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated Parkinsonian monkeys.J Comp Neurol. 2011 Apr 1;519(5):989-1005. doi: 10.1002/cne.22563. J Comp Neurol. 2011. PMID: 21280048 Free PMC article.
-
Loss and remodeling of striatal dendritic spines in Parkinson's disease: from homeostasis to maladaptive plasticity?J Neural Transm (Vienna). 2018 Mar;125(3):431-447. doi: 10.1007/s00702-017-1735-6. Epub 2017 May 24. J Neural Transm (Vienna). 2018. PMID: 28540422 Free PMC article. Review.
-
Striatal spine plasticity in Parkinson's disease.Front Neuroanat. 2010 Dec 10;4:133. doi: 10.3389/fnana.2010.00133. eCollection 2010. Front Neuroanat. 2010. PMID: 21179580 Free PMC article.
-
Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine?Neuroscience. 2013 Oct 22;251:2-20. doi: 10.1016/j.neuroscience.2013.07.011. Epub 2013 Jul 16. Neuroscience. 2013. PMID: 23867772 Free PMC article. Review.
-
Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism.Eur J Neurosci. 2008 Apr;27(7):1647-58. doi: 10.1111/j.1460-9568.2008.06136.x. Eur J Neurosci. 2008. PMID: 18380666
Cited by
-
Acute and protracted abstinence from methamphetamine bidirectionally changes intrinsic excitability of indirect pathway spiny projection neurons in the dorsomedial striatum.Sci Rep. 2022 Jul 15;12(1):12116. doi: 10.1038/s41598-022-16272-6. Sci Rep. 2022. PMID: 35840639 Free PMC article.
-
Striatal molecular signature of subchronic subthalamic nucleus high frequency stimulation in parkinsonian rat.PLoS One. 2013 Apr 4;8(4):e60447. doi: 10.1371/journal.pone.0060447. Print 2013. PLoS One. 2013. PMID: 23593219 Free PMC article.
-
Human striatal recordings reveal abnormal discharge of projection neurons in Parkinson's disease.Proc Natl Acad Sci U S A. 2016 Aug 23;113(34):9629-34. doi: 10.1073/pnas.1606792113. Epub 2016 Aug 8. Proc Natl Acad Sci U S A. 2016. PMID: 27503874 Free PMC article.
-
Neuroglial plasticity at striatal glutamatergic synapses in Parkinson's disease.Front Syst Neurosci. 2011 Aug 23;5:68. doi: 10.3389/fnsys.2011.00068. eCollection 2011. Front Syst Neurosci. 2011. PMID: 21897810 Free PMC article.
-
Cortico-basal ganglia plasticity in motor learning.Neuron. 2024 Aug 7;112(15):2486-2502. doi: 10.1016/j.neuron.2024.06.014. Epub 2024 Jul 12. Neuron. 2024. PMID: 39002543 Free PMC article. Review.
References
-
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The corticobasal ganglia-thalamo-cortical loop. Brain Res Rev. 1996;20:91–127. - PubMed
-
- Smith Y, Shink E, Bevan MD, Bolam JP. Synaptology of the direct and indirect striatofugal pathways. Neuroscience. 1998;86:353–387. - PubMed
-
- Gerfen CR, Wilson CJ. The basal ganglia. In: Björklund A, Hökfelt T, Swanson L, editors. Handbook of Chemical Neuroanatomy, Integrated Systems of the CNS, Part III. Amsterdam: Elsevier; 1996. pp. 369–466.
-
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. - PubMed
-
- Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;19:417–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

