Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade
- PMID: 20083042
- PMCID: PMC2819840
- DOI: 10.1016/S1474-4422(09)70299-6
Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade
Abstract
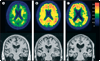
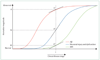
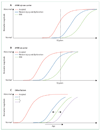
Currently available evidence strongly supports the position that the initiating event in Alzheimer's disease (AD) is related to abnormal processing of beta-amyloid (Abeta) peptide, ultimately leading to formation of Abeta plaques in the brain. This process occurs while individuals are still cognitively normal. Biomarkers of brain beta-amyloidosis are reductions in CSF Abeta(42) and increased amyloid PET tracer retention. After a lag period, which varies from patient to patient, neuronal dysfunction and neurodegeneration become the dominant pathological processes. Biomarkers of neuronal injury and neurodegeneration are increased CSF tau and structural MRI measures of cerebral atrophy. Neurodegeneration is accompanied by synaptic dysfunction, which is indicated by decreased fluorodeoxyglucose uptake on PET. We propose a model that relates disease stage to AD biomarkers in which Abeta biomarkers become abnormal first, before neurodegenerative biomarkers and cognitive symptoms, and neurodegenerative biomarkers become abnormal later, and correlate with clinical symptom severity.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CRJ is an investigator in clinical trials sponsored by Pfizer and Baxter, and consults for Elan and Lilly. DSK has served on a data and safety monitoring board for Lilly, has been a consultant for GlaxoSmithKline, and receives grant funding for clinical trials by Baxter, Elan, and Forest. WJJ has consulted for Genentech, Synarc, Elan Pharmaceuticals, Ceregene, Schering Plough, Merck & Co, and Lilly. LMS has consulted for Pfizer. PSA has consulted for Elan, Wyeth, Eisai, Neurochem, Schering-Plough, Bristol-Myers-Squibb, Lilly, Neurophase, Merck, Roche, Amgen, Genentech, Abbott, Pfizer, Novartis, and Medivation, has stock options with Medivation and Neurophase, and has received grant support from Pfizer, Baxter, and Neuro-Hitech. MWW has been on the scientific advisory boards for the Alzheimer’s Study Group, Bayer Schering Pharma, Lilly, CoMentis, Neurochem, Eisai, Avid, Aegis, Genentech, Allergan, Bristol-Myers Squibb, Forest, Pfizer, McKinsey, Mitsubishi, and Novartis, has received travel funding from Nestlé, honoraria from BOLT International, commercial research support from Merck and Avid, and has stock options in Synarc and Elan. RCP has been a chair, member of the safety monitoring committee, and consultant for Elan, has chaired a data monitoring committee for Wyeth, and has been a consultant for GE Healthcare. JQT has received lecture honoraria from Wyeth, Pfizer, and Takeda.
Figures


References
-
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. - PubMed
-
- White L, Small BJ, Petrovitch H, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18:224–227. - PubMed
-
- Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. - PubMed
-
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. - PubMed
-
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

