mTOR regulation of autophagy
- PMID: 20083114
- PMCID: PMC2846630
- DOI: 10.1016/j.febslet.2010.01.017
mTOR regulation of autophagy
Abstract
Nutrient starvation induces autophagy in eukaryotic cells through inhibition of TOR (target of rapamycin), an evolutionarily-conserved protein kinase. TOR, as a central regulator of cell growth, plays a key role at the interface of the pathways that coordinately regulate the balance between cell growth and autophagy in response to nutritional status, growth factor and stress signals. Although TOR has been known as a key regulator of autophagy for more than a decade, the underlying regulatory mechanisms have not been clearly understood. This review discusses the recent advances in understanding of the mechanism by which TOR regulates autophagy with focus on mammalian TOR (mTOR) and its regulation of the autophagy machinery.
Copyright 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures
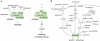

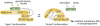
Similar articles
-
AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1.Nat Cell Biol. 2011 Feb;13(2):132-41. doi: 10.1038/ncb2152. Epub 2011 Jan 23. Nat Cell Biol. 2011. PMID: 21258367 Free PMC article.
-
Termination of autophagy and reformation of lysosomes regulated by mTOR.Nature. 2010 Jun 17;465(7300):942-6. doi: 10.1038/nature09076. Epub 2010 Jun 6. Nature. 2010. PMID: 20526321 Free PMC article.
-
The Tor and cAMP-dependent protein kinase signaling pathways coordinately control autophagy in Saccharomyces cerevisiae.Autophagy. 2010 Feb;6(2):294-5. doi: 10.4161/auto.6.2.11129. Epub 2010 Feb 6. Autophagy. 2010. PMID: 20087062
-
LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review.
-
With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging.Cell Metab. 2010 Jun 9;11(6):453-65. doi: 10.1016/j.cmet.2010.05.001. Cell Metab. 2010. PMID: 20519118 Free PMC article. Review.
Cited by
-
DHX15 Inhibits Autophagy and the Proliferation of Hepatoma Cells.Front Med (Lausanne). 2021 Feb 11;7:591736. doi: 10.3389/fmed.2020.591736. eCollection 2020. Front Med (Lausanne). 2021. PMID: 33644083 Free PMC article.
-
The complexity of p53-mediated metabolic regulation in tumor suppression.Semin Cancer Biol. 2022 Oct;85:4-32. doi: 10.1016/j.semcancer.2021.03.010. Epub 2021 Mar 27. Semin Cancer Biol. 2022. PMID: 33785447 Free PMC article. Review.
-
2,3,5,4'-tetrahydroxystilbene-2-O-β-D-glucoside ameliorates bleomycin-induced pulmonary fibrosis via regulating pro-fibrotic signaling pathways.Front Pharmacol. 2022 Oct 4;13:997100. doi: 10.3389/fphar.2022.997100. eCollection 2022. Front Pharmacol. 2022. PMID: 36267283 Free PMC article.
-
Aerosol delivery of beclin1 enhanced the anti-tumor effect of radiation in the lungs of K-rasLA1 mice.J Radiat Res. 2012 Jul;53(4):506-15. doi: 10.1093/jrr/rrs005. Epub 2012 Jun 6. J Radiat Res. 2012. PMID: 22843615 Free PMC article.
-
Cell-autonomous stress responses in innate immunity.J Leukoc Biol. 2017 Jan;101(1):77-86. doi: 10.1189/jlb.2MR0416-201R. Epub 2016 Oct 12. J Leukoc Biol. 2017. PMID: 27733577 Free PMC article. Review.
References
-
- Noda T, Ohsumi Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem. 1998;273:3963–6. - PubMed
-
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–78. - PubMed
-
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–9. - PubMed
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. - PubMed
-
- Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

