Calcium sensitivity, force frequency relationship and cardiac troponin I: critical role of PKA and PKC phosphorylation sites
- PMID: 20083117
- PMCID: PMC2854165
- DOI: 10.1016/j.yjmcc.2010.01.004
Calcium sensitivity, force frequency relationship and cardiac troponin I: critical role of PKA and PKC phosphorylation sites
Abstract
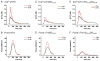
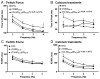
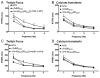
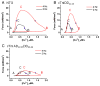
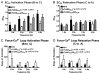
Transgenic models with pseudo phosphorylation mutants of troponin I, PKA sites at Ser 22 and 23 (cTnIDD(22,23) mice) or PKC sites at Ser 42 and 44 (cTnIAD(22,23)DD(42,44)) displayed differential force-frequency relationships and afterload relaxation delay in vivo. We hypothesized that cTnI PKA and PKC phosphomimics impact cardiac muscle rate-related developed twitch force and relaxation kinetics in opposite directions. cTnIDD(22,23) transgenic mice produce a force frequency relationship (FFR) equivalent to control NTG albeit at lower peak [Ca(2+)](i), while cTnIAD(22,23)DD(42,44) TG mice had a flat FFR with normal peak systolic [Ca(2+)](i), thus suggestive of diminished responsiveness to [Ca(2+)](i) at higher frequencies. Force-[Ca(2+)](i) hysteresis analysis revealed that cTnIDD(22,23) mice have a combined enhanced myofilament calcium peak response with an enhanced slope of force development and decline per unit of [Ca(2+)](i), whereas cTnIAD(22,23)DD(42,44) transgenic mice showed the opposite. The computational ECME model predicts that the TG lines may be distinct from each other due to different rate constants for association/dissociation of Ca(2+) at the regulatory site of cTnC. Our data indicate that cTnI phosphorylation at PKA sites plays a critical role in the FFR by increasing relative myofilament responsiveness, and results in a distinctive transition between activation and relaxation, as displayed by force-[Ca(2+)](i) hysteresis loops. These findings may have important implications for understanding the specific contribution of cTnI to beta-adrenergic inotropy and lusitropy and to adverse contractile effects of PKC activation, which is relevant during heart failure development.
Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Figures
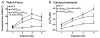
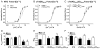

Similar articles
-
Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium.J Physiol. 2003 Nov 1;552(Pt 3):845-57. doi: 10.1113/jphysiol.2003.045260. Epub 2003 Aug 15. J Physiol. 2003. PMID: 12923217 Free PMC article.
-
Distinct sarcomeric substrates are responsible for protein kinase D-mediated regulation of cardiac myofilament Ca2+ sensitivity and cross-bridge cycling.J Biol Chem. 2010 Feb 19;285(8):5674-82. doi: 10.1074/jbc.M109.066456. Epub 2009 Dec 17. J Biol Chem. 2010. PMID: 20018870 Free PMC article.
-
Cardiac Myosin-binding Protein C and Troponin-I Phosphorylation Independently Modulate Myofilament Length-dependent Activation.J Biol Chem. 2015 Dec 4;290(49):29241-9. doi: 10.1074/jbc.M115.686790. Epub 2015 Oct 9. J Biol Chem. 2015. PMID: 26453301 Free PMC article.
-
Regulation of cardiac contractile function by troponin I phosphorylation.Cardiovasc Res. 2005 Apr 1;66(1):12-21. doi: 10.1016/j.cardiores.2004.12.022. Cardiovasc Res. 2005. PMID: 15769444 Review.
-
Suppression of lusitropy as a disease mechanism in cardiomyopathies.Front Cardiovasc Med. 2023 Jan 9;9:1080965. doi: 10.3389/fcvm.2022.1080965. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36698941 Free PMC article. Review.
Cited by
-
Role of RONS and eIFs in Cancer Progression.Oxid Med Cell Longev. 2021 Jul 5;2021:5522054. doi: 10.1155/2021/5522054. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34285764 Free PMC article. Review.
-
Multiple reaction monitoring to identify site-specific troponin I phosphorylated residues in the failing human heart.Circulation. 2012 Oct 9;126(15):1828-37. doi: 10.1161/CIRCULATIONAHA.112.096388. Epub 2012 Sep 12. Circulation. 2012. PMID: 22972900 Free PMC article.
-
Arg92Leu-cTnT Alters the cTnC-cTnI Interface Disrupting PKA-Mediated Relaxation.Circ Res. 2024 Oct 25;135(10):974-989. doi: 10.1161/CIRCRESAHA.124.325223. Epub 2024 Sep 27. Circ Res. 2024. PMID: 39328062
-
TRPC channels blockade abolishes endotoxemic cardiac dysfunction by hampering intracellular inflammation and Ca2+ leakage.Nat Commun. 2022 Dec 2;13(1):7455. doi: 10.1038/s41467-022-35242-0. Nat Commun. 2022. PMID: 36460692 Free PMC article.
-
Regulation of Papillary Muscle Contractility by NAD and Ammonia Interplay: Contribution of Ion Channels and Exchangers.Membranes (Basel). 2022 Dec 7;12(12):1239. doi: 10.3390/membranes12121239. Membranes (Basel). 2022. PMID: 36557146 Free PMC article.
References
-
- Allen DG, Blinks JR. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–13. - PubMed
-
- Layland J, Kentish JC. Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. Am J Physiol. 1999 Jan;276(1 Pt 2):H9–H18. - PubMed
-
- Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol. 2004 Oct 1;500(1–3):73–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

