Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants
- PMID: 20083530
- PMCID: PMC2951128
- DOI: 10.1542/peds.2009-1112
Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants
Abstract
Objective: Antiretroviral (ARV) drugs are routinely provided to HIV-infected pregnant women to prevent HIV mother-to-child transmission. Although ARV use has significantly reduced mother-to-child transmission to <2% in the United States, it remains crucial to monitor uninfected infants and children for adverse consequences of in utero ARV exposure.
Methods: We studied neurodevelopmental function in HIV-exposed uninfected children who were enrolled in Pediatric AIDS Clinical Trials Group 219/219C, a multisite, prospective, cohort study. Mental and motor functioning were assessed with the Bayley Scales of Infant Development (BSID), first and second editions. ARV exposure information was collected during pregnancy or within the first years of life. Linear regression methods were used to evaluate the association of in utero ARV exposure on Mental Developmental Index and Psychomotor Developmental Index at 2 years of age, controlling for demographic factors (age, gender, and race/ethnicity) and potential confounders: test version, primary language, primary caregiver, caregiver education level, low birth weight, geographic and urban/rural location, birth year, and maternal illicit drug use.
Results: Among 1840 infants who were born between 1993 and 2006, 1694 (92%) were exposed to ARV in utero and 146 (8%) were not exposed. After controlling for confounders, children who were exposed in utero to any ARV did not have lower Mental Developmental Index and Psychomotor Developmental Index scores than unexposed children. Among low birth weight infants, significantly higher BSID scores were observed for prenatally ARV-exposed than unexposed children. Maternal illicit drug use was reported for 17% of mothers but was not associated with BSID scores.
Conclusions: Mental and motor functioning scores were not lower for infants with in utero ARV exposure compared with no exposure. Although these results are reassuring, continued evaluation of uninfected children with in utero ARV exposure for long-term adverse outcomes is important.
Figures
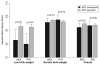
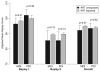
References
-
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331(18):1173–1180. - PubMed
-
- Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1 infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29(5):484–494. - PubMed
-
- Ioannidis JP, Abrams EJ, Ammann A, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads < 1000 copies/ml. J Infect Dis. 2001;183(4):539–545. - PubMed
-
- Suksomboon N, Poolsup N, Ket-aim S. Systematic review of the efficacy of antiretroviral therapies for reducing the risk of mother-to-child transmission of HIV infection. J Clin Pharm Ther. 2007;32(3):293–311. - PubMed
-
- Perinatal HIV Guidelines Working Group Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1 infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. Apr 29, 2009. pp. 1–90. Available at http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. Accessed November .... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

