Intein-mediated cyclization of bacterial acyl carrier protein stabilizes its folded conformation but does not abolish function
- PMID: 20083605
- PMCID: PMC2838282
- DOI: 10.1074/jbc.M109.060863
Intein-mediated cyclization of bacterial acyl carrier protein stabilizes its folded conformation but does not abolish function
Abstract
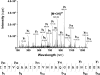
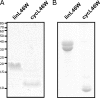
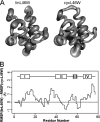
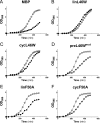
Bacterial acyl carrier protein (ACP) is essential for the synthesis of fatty acids and serves as the major acyl donor for the formation of phospholipids and other lipid products. Acyl-ACP encloses attached fatty acyl groups in a hydrophobic pocket within a four-helix bundle, but must at least partially unfold to present the acyl chain to the active sites of its multiple enzyme partners. To further examine the constraints of ACP structure and function, we have constructed a cyclic version of Vibrio harveyi ACP, using split-intein technology to covalently join its closely apposed N and C termini. Cyclization stabilized ACP in a folded helical conformation as indicated by gel electrophoresis, circular dichroism, fluorescence, and mass spectrometry. Molecular dynamics simulations also indicated overall decreased polypeptide chain mobility in cyclic ACP, although no major conformational rearrangements over a 10-ns period were noted. In vivo complementation assays revealed that cyclic ACP can functionally replace the linear wild-type protein and support growth of an Escherichia coli ACP-null mutant strain. Cyclization of a folding-deficient ACP mutant (F50A) both restored its ability to adopt a folded conformation and enhanced complementation of growth. Our results thus suggest that ACP must be able to adopt a folded conformation for biological activity, and that its function does not require complete unfolding of the protein.
Figures



Similar articles
-
NMR solution structure and biophysical characterization of Vibrio harveyi acyl carrier protein A75H: effects of divalent metal ions.J Biol Chem. 2010 Oct 1;285(40):30558-66. doi: 10.1074/jbc.M110.128298. Epub 2010 Jul 21. J Biol Chem. 2010. PMID: 20659901 Free PMC article.
-
Site-directed mutagenesis of acyl carrier protein (ACP) reveals amino acid residues involved in ACP structure and acyl-ACP synthetase activity.J Biol Chem. 2001 Sep 21;276(38):35934-9. doi: 10.1074/jbc.M101849200. Epub 2001 Jul 6. J Biol Chem. 2001. PMID: 11443113
-
Neutralization of acidic residues in helix II stabilizes the folded conformation of acyl carrier protein and variably alters its function with different enzymes.J Biol Chem. 2007 Feb 16;282(7):4494-4503. doi: 10.1074/jbc.M608234200. Epub 2006 Dec 18. J Biol Chem. 2007. PMID: 17179150
-
Acyl carrier protein: structure-function relationships in a conserved multifunctional protein family.Biochem Cell Biol. 2007 Dec;85(6):649-62. doi: 10.1139/o07-109. Biochem Cell Biol. 2007. PMID: 18059524 Review.
-
The chain-flipping mechanism of ACP (acyl carrier protein)-dependent enzymes appears universal.Biochem J. 2014 Jun 1;460(2):157-63. doi: 10.1042/BJ20140239. Biochem J. 2014. PMID: 24825445 Review.
Cited by
-
Recombinant expression of backbone-cyclized polypeptides.Biopolymers. 2013 Sep;100(5):502-9. doi: 10.1002/bip.22306. Biopolymers. 2013. PMID: 23893781 Free PMC article. Review.
-
Acyl Carrier Protein 3 Is Involved in Oxidative Stress Response in Pseudomonas aeruginosa.Front Microbiol. 2018 Sep 20;9:2244. doi: 10.3389/fmicb.2018.02244. eCollection 2018. Front Microbiol. 2018. PMID: 30294316 Free PMC article.
-
Cyclization of Short Peptides Designed from Late Embryogenesis Abundant Protein to Improve Stability and Functionality.Chembiochem. 2025 Apr 14;26(8):e202401013. doi: 10.1002/cbic.202401013. Epub 2025 Feb 20. Chembiochem. 2025. PMID: 39912732 Free PMC article.
-
Intein-mediated recombinant expression of monomeric B22Asp desB30 insulin.BMC Biotechnol. 2020 Jan 9;20(1):3. doi: 10.1186/s12896-020-0598-3. BMC Biotechnol. 2020. PMID: 31918694 Free PMC article.
-
The conserved modular elements of the acyl carrier proteins of lipid synthesis are only partially interchangeable.J Biol Chem. 2015 May 29;290(22):13791-9. doi: 10.1074/jbc.M115.648402. Epub 2015 Apr 10. J Biol Chem. 2015. PMID: 25861991 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

