CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/glycogen synthase kinase-3beta/beta-catenin signaling
- PMID: 20083607
- PMCID: PMC2843172
- DOI: 10.1074/jbc.M109.043711
CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/glycogen synthase kinase-3beta/beta-catenin signaling
Erratum in
-
Correction: CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/glycogen synthase kinase-3β/β-catenin signaling.J Biol Chem. 2020 Mar 6;295(10):3388. doi: 10.1074/jbc.AAC120.013006. J Biol Chem. 2020. PMID: 32144151 Free PMC article. No abstract available.
Abstract
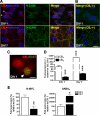
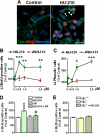
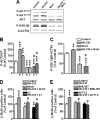
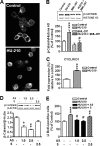
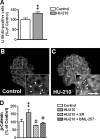
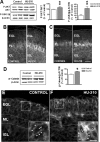
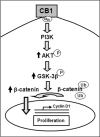
The endocannabinoid system is involved in the regulation of many physiological effects in the central and peripheral nervous system. Recent findings have demonstrated the presence of a functional endocannabinoid system within neuronal progenitors located in the hippocampus and ventricular/subventricular zone that participates in the regulation of cell proliferation. It is presently unknown whether the endocannabinoid system exerts a widespread effect on neuronal precursors from different neurogenic regions, and very little is known about the signaling by which it regulates neuronal precursor proliferation. Herein, we demonstrate the presence of cannabinoid CB(1) receptors in granule cell precursors (GCPs) during early cerebellar development. Activation of CB(1) receptors by HU-210 promoted GCP proliferation in vitro, an effect that was prevented by a selective CB(1) antagonist. Accordingly, in vivo experiments showed that GCP proliferation was increased by chronic HU-210 treatment and that in CB(1)-deficient mice cell proliferation was significantly lower than in wild-type littermates, indicating that the endocannabinoid system is physiologically involved in regulation of GCP proliferation. The pro-proliferative effect of cannabinoids in GCPs was mediated through the CB(1)/AKT/glycogen synthase kinase-3beta/beta-catenin pathway. Involvement of this pathway was also observed in cultures of neuronal precursors from the subventricular zone, suggesting that this pathway may be a general mechanism by which endocannabinoids regulate proliferation of neuronal precursors. These observations suggest that endocannabinoids constitute a new family of lipid signaling cues that may exert a widespread effect on neuronal precursor proliferation during brain development.
Figures

References
-
- Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. (1990) Nature 346, 561–564 - PubMed
-
- Munro S., Thomas K. L., Abu-Shaar M. (1993) Nature 365, 61–65 - PubMed
-
- Piomelli D., Giuffrida A., Calignano A., Rodríguez de Fonseca F. (2000) Trends Pharmacol. Sci. 21, 218–224 - PubMed
-
- Porter A. C., Felder C. C. (2001) Pharmacol. Ther. 90, 45–60 - PubMed
-
- Di Marzo V., Melck D., Bisogno T., De Petrocellis L. (1998) Trends Neurosci. 21, 521–528 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

