Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice
- PMID: 20083666
- PMCID: PMC2887735
- DOI: 10.4049/jimmunol.0902778
Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice
Abstract
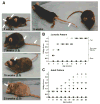
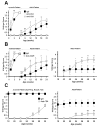
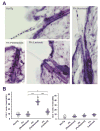
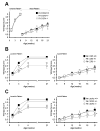
Generalized vitiligo is thought to have an autoimmune etiology and has been correlated with the presence of CD8 T cells specific for melanocyte differentiation Ag. However, limited animal models for the disease have hampered its understanding. Thus, we generated TCR transgenic mice that recognize an epitope of the melanocyte protein, tyrosinase. These animals develop vitiligo with strikingly similar characteristics to the human disease. Vitiligo develops temporally and spatially, with juvenile lesions forming bilaterally in head and facial areas, and only arising later in the body of adult animals. Vitiligo is entirely dependent on CD8 T cells, whereas CD4 T cells exert a negative regulatory effect. Importantly, CD8 T cells can be pervasively present in the skin in the steady state without inducing vitiligo in most areas. This points to developmental differences in melanocyte susceptibility and/or immunological effector mechanisms over time, or in different body locations. Disease is strongly dependent on both IFN-gamma and CXCR3, whereas dependence on CCR5 is more limited, and both CCR4 and perforin are dispensable. Genetic ablation of CXCR3 or IFN-gamma also resulted in scarce CD8 T cell infiltration into the skin. Our results identify unexpected complexity in vitiligo development and point toward possible therapeutic interventions.
Conflict of interest statement
Figures


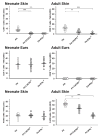
References
-
- Handa S, Kaur I. Vitiligo: clinical findings in 1436 patients. J Dermatol. 1999;26:653–657. - PubMed
-
- Huggins RH, Schwartz RA, Janniger CK. Vitiligo. Acta Dermatovenerol Alp Panonica Adriat. 2005;14:137–142. - PubMed
-
- Taieb A, Picardo M. Clinical practice. Vitiligo. N Engl J Med. 2009;360:160–169. - PubMed
-
- Palermo B, Campanelli R, Garbelli S, Mantovani S, Lantelme E, Brazzelli V, Ardigo M, Borroni G, Martinetti M, Badulli C, Necker A, Giachino C. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol. 2001;117:326–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 CA121916/CA/NCI NIH HHS/United States
- CA78400/CA/NCI NIH HHS/United States
- T32 AI007496/AI/NIAID NIH HHS/United States
- S06 GM008136/GM/NIGMS NIH HHS/United States
- AI068836/AI/NIAID NIH HHS/United States
- R01 AI068836/AI/NIAID NIH HHS/United States
- GM08136/GM/NIGMS NIH HHS/United States
- AI07486/AI/NIAID NIH HHS/United States
- T32 AI007046/AI/NIAID NIH HHS/United States
- R01 CA078400/CA/NCI NIH HHS/United States
- CA121916/CA/NCI NIH HHS/United States
- R21 AI059996/AI/NIAID NIH HHS/United States
- AI059996/AI/NIAID NIH HHS/United States
- A107486/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

