Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice
- PMID: 20083684
- PMCID: PMC2836805
- DOI: 10.1161/CIRCULATIONAHA.109.862946
Failure of postnatal ductus arteriosus closure in prostaglandin transporter-deficient mice
Abstract
Background: Prostaglandin E(2) (PGE(2)) plays a major role both in maintaining patency of the fetal ductus arteriosus and in closure of the ductus arteriosus after birth. The rate-limiting step in PGE(2) signal termination is PGE(2) uptake by the transporter PGT.
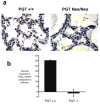
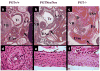
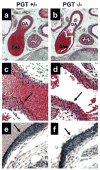
Methods and results: To determine the role of PGT in ductus arteriosus closure, we used a gene-targeting strategy to produce mice in which PGT exon 1 was flanked by loxP sites. Successful targeting was obtained because neither mice hypomorphic at the PGT allele (PGT Neo/Neo) nor global PGT knockout mice (PGT(-/-)) exhibited PGT protein expression; moreover, embryonic fibroblasts isolated from targeted mice failed to exhibit carrier-mediated PGE(2) uptake. Although born in a normal mendelian ratio, no PGT(-/-) mice survived past postnatal day 1, and no PGT Neo/Neo mice survived past postnatal day 2. Necropsy revealed patent ductus arteriosus with normal intimal thickening but dilated cardiac chambers. Both PGT Neo/Neo and PGT(-/-) mice could be rescued through the postnatal period by giving the mother indomethacin before birth. Rescued mice grew normally and had no abnormalities by gross and microscopic postmortem analyses. In accordance with the known role of PGT in metabolizing PGE(2), rescued adult PGT(-/-) mice had lower plasma PGE(2) metabolite levels and higher urinary PGE(2) excretion rates than wild-type mice.
Conclusions: PGT plays a critical role in closure of the ductus arteriosus after birth by ensuring a reduction in local and/or circulating PGE(2) concentrations.
Figures




Similar articles
-
Development of a high-affinity inhibitor of the prostaglandin transporter.J Pharmacol Exp Ther. 2011 Nov;339(2):633-41. doi: 10.1124/jpet.111.181354. Epub 2011 Aug 17. J Pharmacol Exp Ther. 2011. PMID: 21849625 Free PMC article.
-
Role of prostaglandin E and its receptors in the process of ductus arteriosus maturation and functional closure in the rabbit.Clin Exp Pharmacol Physiol. 2010 May;37(5-6):574-80. doi: 10.1111/j.1440-1681.2010.05354.x. Epub 2010 Jan 17. Clin Exp Pharmacol Physiol. 2010. PMID: 20082631
-
Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice.Biochem Biophys Res Commun. 1998 May 8;246(1):7-12. doi: 10.1006/bbrc.1998.8461. Biochem Biophys Res Commun. 1998. PMID: 9600059
-
Molecular Mechanisms Underlying Remodeling of Ductus Arteriosus: Looking beyond the Prostaglandin Pathway.Int J Mol Sci. 2021 Mar 22;22(6):3238. doi: 10.3390/ijms22063238. Int J Mol Sci. 2021. PMID: 33810164 Free PMC article. Review.
-
Prostaglandin E-mediated molecular mechanisms driving remodeling of the ductus arteriosus.Pediatr Int. 2015 Oct;57(5):820-7. doi: 10.1111/ped.12769. Pediatr Int. 2015. PMID: 26228894 Review.
Cited by
-
Inhibition of the Prostaglandin Transporter PGT Lowers Blood Pressure in Hypertensive Rats and Mice.PLoS One. 2015 Jun 29;10(6):e0131735. doi: 10.1371/journal.pone.0131735. eCollection 2015. PLoS One. 2015. PMID: 26121580 Free PMC article.
-
Chloride Channels in Astrocytes: Structure, Roles in Brain Homeostasis and Implications in Disease.Int J Mol Sci. 2019 Feb 27;20(5):1034. doi: 10.3390/ijms20051034. Int J Mol Sci. 2019. PMID: 30818802 Free PMC article. Review.
-
Contribution of Prostaglandin Transporter OATP2A1/SLCO2A1 to Placenta-to-Maternal Hormone Signaling and Labor Induction.iScience. 2020 May 22;23(5):101098. doi: 10.1016/j.isci.2020.101098. Epub 2020 Apr 27. iScience. 2020. PMID: 32408168 Free PMC article.
-
The Prostaglandin Transporter: Eicosanoid Reuptake, Control of Signaling, and Development of High-Affinity Inhibitors as Drug Candidates.Trans Am Clin Climatol Assoc. 2015;126:248-57. Trans Am Clin Climatol Assoc. 2015. PMID: 26330684 Free PMC article. Review.
-
A novel mutation in the SLCO2A1 gene, encoding a prostaglandin transporter, induces chronic enteropathy.PLoS One. 2020 Nov 9;15(11):e0241869. doi: 10.1371/journal.pone.0241869. eCollection 2020. PLoS One. 2020. PMID: 33166338 Free PMC article.
References
-
- Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37–49. - PubMed
-
- Schneider DJ, Moore JW. Patent ductus arteriosus. Circulation. 2006;114:1873–1882. - PubMed
-
- Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Matsuoka T, Ushikubi F, Hirose M, Tanaka T, Yoshida N, Narumiya S, Ichikawa A. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. - PubMed
-
- Loftin CD, Trivedi DB, Tiano HF, Clark JA, Lee CA, Epstein JA, Morham SG, Breyer MD, Nguyen M, Hawkins BM, Goulet JL, Smithies O, Koller BH, Langenbach R. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1059–1064. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

