Atg7 deficiency increases resistance of MCF-7 human breast cancer cells to photodynamic therapy
- PMID: 20083906
- PMCID: PMC4459572
- DOI: 10.4161/auto.6.2.11077
Atg7 deficiency increases resistance of MCF-7 human breast cancer cells to photodynamic therapy
Abstract
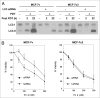
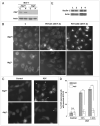
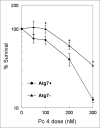
Photodynamic therapy (PDT) uses a photosensitizer, light and oxygen to produce extensive oxidative damage to organelles housing the photosensitizer. Although PDT is an efficient trigger of apoptosis, it also induces autophagy in many kinds of cells. Autophagy can serve as both a cell survival and a cell death mechanism. Our previous study indicates that autophagy contributes to cell death after PDT, especially in apoptosis-deficient cells. Here, we provide further evidence to support the role of autophagy in cell killing after PDT. Autophagy was blocked by knockdown of one essential factor, LC3 or Atg7, in MCF-7 cells. The cells were exposed to a range of doses of PDT sensitized by the phthalocyanine Pc 4; steps in autophagy were monitored by western blotting for LC3-II and by fluorescence microscopy for the uptake of monodansylcadaverine or for the distribution of transfected GFP-LC3; and overall cell death was monitored by MTT assay and by clonogenic assay. We find that blocking autophagy increased the survival of MCF-7 cells after PDT and increased the shoulder on the dose-response curve. In response to Pc 4-PDT, Atg7-deficient MCF-7 cells remained capable of robust accumulation of LC3-II, but were defective in comparison to Atg7(+) cells in the formation of autophagosomes. We conclude that apoptosis-deficient cells rely on autophagy for cell death after Pc 4-PDT and that the strong activation of LC3 maturation in response to PDT could occur even in cells with limited or no Atg7 expression.
Figures

Similar articles
-
ATG7 deficiency suppresses apoptosis and cell death induced by lysosomal photodamage.Autophagy. 2012 Sep;8(9):1333-41. doi: 10.4161/auto.20792. Epub 2012 Aug 14. Autophagy. 2012. PMID: 22889762 Free PMC article.
-
Photodynamic therapy with the novel photosensitizer chlorophyllin f induces apoptosis and autophagy in human bladder cancer cells.Lasers Surg Med. 2014 Apr;46(4):319-34. doi: 10.1002/lsm.22225. Epub 2014 Jan 24. Lasers Surg Med. 2014. PMID: 24464873
-
Increased ceramide accumulation correlates with downregulation of the autophagy protein ATG-7 in MCF-7 cells sensitized to photodamage.Arch Biochem Biophys. 2010 Feb 1;494(1):101-5. doi: 10.1016/j.abb.2009.11.023. Epub 2009 Nov 26. Arch Biochem Biophys. 2010. PMID: 19944062 Free PMC article.
-
Ready player one? Autophagy shapes resistance to photodynamic therapy in cancers.Apoptosis. 2018 Dec;23(11-12):587-606. doi: 10.1007/s10495-018-1489-0. Apoptosis. 2018. PMID: 30288638 Review.
-
Assessing autophagy in the context of photodynamic therapy.Autophagy. 2010 Jan;6(1):7-18. doi: 10.4161/auto.6.1.10220. Epub 2010 Jan 1. Autophagy. 2010. PMID: 19855190 Free PMC article. Review.
Cited by
-
Current Challenges and Opportunities of Photodynamic Therapy against Cancer.Pharmaceutics. 2023 Jan 18;15(2):330. doi: 10.3390/pharmaceutics15020330. Pharmaceutics. 2023. PMID: 36839652 Free PMC article. Review.
-
Apoptotic and autophagic responses to photodynamic therapy in 1c1c7 murine hepatoma cells.Autophagy. 2011 Sep;7(9):979-84. doi: 10.4161/auto.7.9.15865. Epub 2011 Sep 1. Autophagy. 2011. PMID: 21555918 Free PMC article.
-
Combinatorial therapeutic approaches with RNAi and anticancer drugs using nanodrug delivery systems.Drug Dev Ind Pharm. 2017 Sep;43(9):1391-1401. doi: 10.1080/03639045.2017.1313861. Epub 2017 May 19. Drug Dev Ind Pharm. 2017. PMID: 28523942 Free PMC article.
-
Single-cell analysis challenges the connection between autophagy and senescence induced by DNA damage.Autophagy. 2015;11(7):1099-113. doi: 10.1080/15548627.2015.1009795. Autophagy. 2015. PMID: 25701485 Free PMC article.
-
Does the Autophagy Related Gene 7 (ATG7) Have a Role in Non-Melanoma Skin Cancer?Clin Cosmet Investig Dermatol. 2020 Jan 20;13:49-58. doi: 10.2147/CCID.S222051. eCollection 2020. Clin Cosmet Investig Dermatol. 2020. PMID: 32021368 Free PMC article.
References
-
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–48. - PubMed
-
- Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
