Pericontusion axon sprouting is spatially and temporally consistent with a growth-permissive environment after traumatic brain injury
- PMID: 20084019
- PMCID: PMC2821052
- DOI: 10.1097/NEN.0b013e3181cb5bee
Pericontusion axon sprouting is spatially and temporally consistent with a growth-permissive environment after traumatic brain injury
Abstract
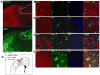
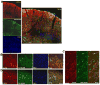
We previously reported that pericontusional extracellular chondroitin sulfate proteoglycans (CSPGs) are profoundly reduced for 3 weeks after experimental traumatic brain injury, indicating a potential growth-permissive window for plasticity. Here, we investigate the extracellular environment of sprouting neurons after controlled cortical impact injury in adult rats to determine the spatial and temporal arrangement of inhibitory and growth-promoting molecules in relation to growth-associated protein 43-positive (GAP43+) neurons. Spontaneous cortical sprouting was maximal in pericontused regions at 7 and 14 days after injury but absent by 28 days. Perineuronal nets containing CSPGs were reduced at 7 days after injury in the pericontused region (p < 0.05), which was commensurate with a reduction in extracellular CSPGs. Sprouting was restricted to the perineuronal nets and CSPG-deficient regions at 7 days, indicating that the pericontused region is temporarily and spatially permissive to new growth. At this time point,GAP43+ neurons were associated with brain regions containing cells positive for polysialic acid neural cell adhesion molecule but not with fibronectin-positive cells. Brain-derived neurotrophic factor was reduced in the immediate pericontused region at 7 days. Along with prior Western blot evidence, these data suggest that a lowered intrinsic growth stimulus, together with a later return of growth-inhibitory CSPGs, may contribute to the ultimate disappearance of sprouting neurons after traumatic brain injury.
Figures






References
-
- Hsu JC, Stein SA, Xu X. Temporal and spatial distribution of growth-associated molecules and astroglial cells in the rat corticospinal tract during development. J Neurosc Res. 2005;80:330–40. - PubMed
-
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–14. - PubMed
-
- Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Curr Opin Neurobiol. 2007;17:516–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

