Kidney development in the absence of Gdnf and Spry1 requires Fgf10
- PMID: 20084103
- PMCID: PMC2797609
- DOI: 10.1371/journal.pgen.1000809
Kidney development in the absence of Gdnf and Spry1 requires Fgf10
Abstract
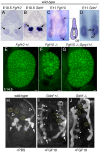
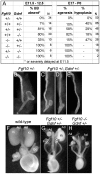
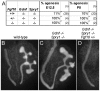
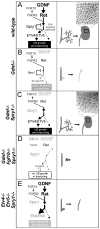
GDNF signaling through the Ret receptor tyrosine kinase (RTK) is required for ureteric bud (UB) branching morphogenesis during kidney development in mice and humans. Furthermore, many other mutant genes that cause renal agenesis exert their effects via the GDNF/RET pathway. Therefore, RET signaling is believed to play a central role in renal organogenesis. Here, we re-examine the extent to which the functions of Gdnf and Ret are unique, by seeking conditions in which a kidney can develop in their absence. We find that in the absence of the negative regulator Spry1, Gdnf, and Ret are no longer required for extensive kidney development. Gdnf-/-;Spry1-/- or Ret-/-;Spry1-/- double mutants develop large kidneys with normal ureters, highly branched collecting ducts, extensive nephrogenesis, and normal histoarchitecture. However, despite extensive branching, the UB displays alterations in branch spacing, angle, and frequency. UB branching in the absence of Gdnf and Spry1 requires Fgf10 (which normally plays a minor role), as removal of even one copy of Fgf10 in Gdnf-/-;Spry1-/- mutants causes a complete failure of ureter and kidney development. In contrast to Gdnf or Ret mutations, renal agenesis caused by concomitant lack of the transcription factors ETV4 and ETV5 is not rescued by removing Spry1, consistent with their role downstream of both RET and FGFRs. This shows that, for many aspects of renal development, the balance between positive signaling by RTKs and negative regulation of this signaling by SPRY1 is more critical than the specific role of GDNF. Other signals, including FGF10, can perform many of the functions of GDNF, when SPRY1 is absent. But GDNF/RET signaling has an apparently unique function in determining normal branching pattern. In contrast to GDNF or FGF10, Etv4 and Etv5 represent a critical node in the RTK signaling network that cannot by bypassed by reducing the negative regulation of upstream signals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
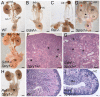
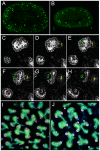
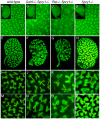

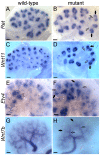

References
-
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. - PubMed
-
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. - PubMed
-
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. - PubMed
-
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. - PubMed
-
- Costantini F, Shakya R. GDNF/Ret signaling and the development of the kidney. Bioessays. 2006;28:117–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

