Effect of leucovorin (folinic acid) on the developmental quotient of children with Down's syndrome (trisomy 21) and influence of thyroid status
- PMID: 20084109
- PMCID: PMC2799517
- DOI: 10.1371/journal.pone.0008394
Effect of leucovorin (folinic acid) on the developmental quotient of children with Down's syndrome (trisomy 21) and influence of thyroid status
Abstract
Background: Seven genes involved in folate metabolism are located on chromosome 21. Previous studies have shown that folate deficiency may contribute to mental retardation in Down's syndrome (DS).
Methodology: We investigated the effect of oral folate supplementation (daily dose of 1.0+/-0.3 mg/kg) on cognitive functions in DS children, aged from 3 to 30 months. They received 1 mg/kg leucovorin or placebo daily, for 12 months, in a single-centre, randomised, double-blind study. Folinic acid (leucovorin, LV) was preferred to folic acid as its bioavailability is higher. The developmental age (DA) of the patients was assessed on the Brunet-Lezine scale, from baseline to the end of treatment.
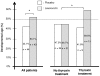
Results: The intent-to-treat analysis (113 patients) did not show a positive effect of leucovorin treatment. However, it identified important factors influencing treatment effect, such as age, sex, and concomitant treatments, including thyroid treatment in particular. A per protocol analysis was carried out on patients evaluated by the same examiner at the beginning and end of the treatment period. This analysis of 87 patients (43 LV-treated vs. 44 patients on placebo) revealed a positive effect of leucovorin on developmental age (DA). DA was 53.1% the normal value with leucovorin and only 44.1% with placebo (p<0.05). This positive effect of leucovorin was particularly strong in patients receiving concomitant thyroxin treatment (59.5% vs. 41.8%, p<0.05). No adverse event related to leucovorin was observed.
Conclusion: These results suggest that leucovorin improves the psychomotor development of children with Down's syndrome, at least in some subgroups of the DS population, particularly those on thyroxin treatment.
Trial registration: ClinicalTrials.gov, NCT00294593.
Conflict of interest statement
Figures

Similar articles
-
Supplementation with antioxidants and folinic acid for children with Down's syndrome: randomised controlled trial.BMJ. 2008 Mar 15;336(7644):594-7. doi: 10.1136/bmj.39465.544028.AE. Epub 2008 Feb 21. BMJ. 2008. PMID: 18296460 Free PMC article. Clinical Trial.
-
Thyroid hormone and folinic acid in young children with Down syndrome: the phase 3 ACTHYF trial.Genet Med. 2020 Jan;22(1):44-52. doi: 10.1038/s41436-019-0597-8. Epub 2019 Jul 8. Genet Med. 2020. PMID: 31281181 Clinical Trial.
-
Safety and efficacy of cognitive training plus epigallocatechin-3-gallate in young adults with Down's syndrome (TESDAD): a double-blind, randomised, placebo-controlled, phase 2 trial.Lancet Neurol. 2016 Jul;15(8):801-810. doi: 10.1016/S1474-4422(16)30034-5. Lancet Neurol. 2016. PMID: 27302362 Clinical Trial.
-
Understanding mental retardation in Down's syndrome using trisomy 16 mouse models.Genes Brain Behav. 2003 Jun;2(3):167-78. doi: 10.1034/j.1601-183x.2003.00024.x. Genes Brain Behav. 2003. PMID: 12931790 Review.
-
Down's syndrome and leukemia: epidemiology, genetics, cytogenetics and mechanisms of leukemogenesis.Cancer Genet Cytogenet. 1987 Sep;28(1):55-76. doi: 10.1016/0165-4608(87)90354-2. Cancer Genet Cytogenet. 1987. PMID: 2955886 Review.
Cited by
-
Heterogeneous RNA editing and influence of ADAR2 on mesothelioma chemoresistance and the tumor microenvironment.Mol Oncol. 2022 Dec;16(22):3949-3974. doi: 10.1002/1878-0261.13322. Epub 2022 Oct 31. Mol Oncol. 2022. PMID: 36221913 Free PMC article.
-
Mouse models of Down syndrome as a tool to unravel the causes of mental disabilities.Neural Plast. 2012;2012:584071. doi: 10.1155/2012/584071. Epub 2012 May 22. Neural Plast. 2012. PMID: 22685678 Free PMC article. Review.
-
Trans-acting epigenetic effects of chromosomal aneuploidies: lessons from Down syndrome and mouse models.Epigenomics. 2017 Feb;9(2):189-207. doi: 10.2217/epi-2016-0138. Epub 2016 Dec 2. Epigenomics. 2017. PMID: 27911079 Free PMC article. Review.
-
Pharmacological approaches to improving cognitive function in Down syndrome: current status and considerations.Drug Des Devel Ther. 2014 Dec 17;9:103-25. doi: 10.2147/DDDT.S51476. eCollection 2015. Drug Des Devel Ther. 2014. PMID: 25552901 Free PMC article. Review.
-
Placebo Responses in Genetically Determined Intellectual Disability: A Meta-Analysis.PLoS One. 2015 Jul 30;10(7):e0133316. doi: 10.1371/journal.pone.0133316. eCollection 2015. PLoS One. 2015. PMID: 26226597 Free PMC article.
References
-
- Lejeune J, Gautier M, Turpin R. Study of somatic chromosomes from 9 mongoloid children. C R Hebd Seances Acad Sci. 1959;248:1721–1722. - PubMed
-
- Epstein CJ. Down syndrome (Trisomy 21). In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 7th ed. New York: McGraw-Hill, Health Professions Division; 1995. pp. 749–794.
-
- Antonarakis SE, Lyle R, Dermitzakis ET, Reymond A, Deutsch S. Chromosome 21 and down syndrome: from genomics to pathophysiology. Nat Rev Genet. 2004;5:725–738. - PubMed
-
- Dauphinot L, Lyle R, Rivals I, Dang MT, Moldrich RX, et al. The cerebellar transcriptome during postnatal development of the Ts1Cje mouse, a segmental trisomy model for Down syndrome. Hum Mol Genet. 2005;14:373–384. - PubMed
-
- Chapman RS, Hesketh LJ. Behavioral phenotype of individuals with Down syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:84–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

