Genetic dissection of differential signaling threshold requirements for the Wnt/beta-catenin pathway in vivo
- PMID: 20084116
- PMCID: PMC2800045
- DOI: 10.1371/journal.pgen.1000816
Genetic dissection of differential signaling threshold requirements for the Wnt/beta-catenin pathway in vivo
Abstract
Contributions of null and hypomorphic alleles of Apc in mice produce both developmental and pathophysiological phenotypes. To ascribe the resulting genotype-to-phenotype relationship unambiguously to the Wnt/beta-catenin pathway, we challenged the allele combinations by genetically restricting intracellular beta-catenin expression in the corresponding compound mutant mice. Subsequent evaluation of the extent of resulting Tcf4-reporter activity in mouse embryo fibroblasts enabled genetic measurement of Wnt/beta-catenin signaling in the form of an allelic series of mouse mutants. Different permissive Wnt signaling thresholds appear to be required for the embryonic development of head structures, adult intestinal polyposis, hepatocellular carcinomas, liver zonation, and the development of natural killer cells. Furthermore, we identify a homozygous Apc allele combination with Wnt/beta-catenin signaling capacity similar to that in the germline of the Apc(min) mice, where somatic Apc loss-of-heterozygosity triggers intestinal polyposis, to distinguish whether co-morbidities in Apc(min) mice arise independently of intestinal tumorigenesis. Together, the present genotype-phenotype analysis suggests tissue-specific response levels for the Wnt/beta-catenin pathway that regulate both physiological and pathophysiological conditions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

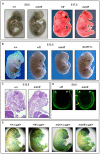




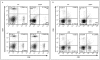
Similar articles
-
A targeted constitutive mutation in the APC tumor suppressor gene underlies mammary but not intestinal tumorigenesis.PLoS Genet. 2009 Jul;5(7):e1000547. doi: 10.1371/journal.pgen.1000547. Epub 2009 Jul 3. PLoS Genet. 2009. PMID: 19578404 Free PMC article.
-
The threshold level of adenomatous polyposis coli protein for mouse intestinal tumorigenesis.Cancer Res. 2005 Oct 1;65(19):8622-7. doi: 10.1158/0008-5472.CAN-05-2145. Cancer Res. 2005. PMID: 16204028
-
APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression.Gastroenterology. 2006 Oct;131(4):1096-109. doi: 10.1053/j.gastro.2006.08.011. Epub 2006 Aug 16. Gastroenterology. 2006. PMID: 17030180
-
Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer.Cells. 2020 Sep 19;9(9):2125. doi: 10.3390/cells9092125. Cells. 2020. PMID: 32961708 Free PMC article. Review.
-
Cytoskeleton out of the cupboard: colon cancer and cytoskeletal changes induced by loss of APC.Nat Rev Cancer. 2006 Dec;6(12):967-74. doi: 10.1038/nrc2010. Epub 2006 Nov 9. Nat Rev Cancer. 2006. PMID: 17093505 Review.
Cited by
-
Sfrp5 modulates both Wnt and BMP signaling and regulates gastrointestinal organogenesis [corrected] in the zebrafish, Danio rerio.PLoS One. 2013 Apr 29;8(4):e62470. doi: 10.1371/journal.pone.0062470. Print 2013. PLoS One. 2013. PMID: 23638093 Free PMC article.
-
β-catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis.Gut. 2011 Sep;60(9):1204-12. doi: 10.1136/gut.2010.233460. Epub 2011 Feb 9. Gut. 2011. PMID: 21307168 Free PMC article.
-
Structural variants in the Epb41l4a locus: TAD disruption and Nrep gene misregulation as hypothetical drivers of neurodevelopmental outcomes.Sci Rep. 2024 Mar 4;14(1):5288. doi: 10.1038/s41598-024-52545-y. Sci Rep. 2024. PMID: 38438377 Free PMC article.
-
A hypermorphic epithelial β-catenin mutation facilitates intestinal tumorigenesis in mice in response to compounding WNT-pathway mutations.Dis Model Mech. 2015 Nov;8(11):1361-73. doi: 10.1242/dmm.019844. Epub 2015 Aug 6. Dis Model Mech. 2015. PMID: 26398937 Free PMC article.
-
Wnt signalling modulates transcribed-ultraconserved regions in hepatobiliary cancers.Gut. 2017 Jul;66(7):1268-1277. doi: 10.1136/gutjnl-2016-312278. Epub 2016 Sep 12. Gut. 2017. PMID: 27618837 Free PMC article.
References
-
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. - PubMed
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
-
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. - PubMed
-
- Satoh W, Gotoh T, Tsunematsu Y, Aizawa S, Shimono A. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006;133:989–999. - PubMed
-
- Popperl H, Schmidt C, Wilson V, Hume CR, Dodd J, et al. Misexpression of Cwnt8C in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development. 1997;124:2997–3005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

