Net cost of palivizumab for respiratory syncytial virus prophylaxis during the 1998/99 season in northern Alberta
- PMID: 20084122
- PMCID: PMC2805588
- DOI: 10.1093/pch/6.8.525
Net cost of palivizumab for respiratory syncytial virus prophylaxis during the 1998/99 season in northern Alberta
Abstract
Objective: Palivizumab has been shown to decrease respiratory syncytial virus (RSV) hospitalization rates in preterm infants and infants with chronic lung disease. The objective of the present study was to determine whether the use of palivizumab during the 1998/99 RSV season would have resulted in a cost-saving in infants discharged from Edmonton hospitals.
Design: A retrospective study of RSV hospitalizations was performed by contacting parents and reviewing hospital lists. The net cost of using palivizumab was determined by comparing the cost of giving the drug from November 1, 1998 to April 1, 1999 with the cost of potentially averted medical transports and hospitalizations.
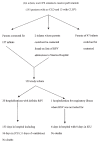
Population: One hundred fifty-nine infants discharged from Edmonton hospitals who met the Canadian Paediatric Society's criteria for receiving palivizumab during the 1998/99 RSV season were studied.
Results: The cost of using palivizumab in these 159 study infants would have been $753,300. The infants had 21 RSV hospitalizations and required four medical transports. The estimated cost of RSV hospital-based care for these infants was $168,888. Assuming a drug efficacy of 39% in infants with chronic lung disease and 78% in infants born before 33 weeks' gestation with no chronic lung disease, $121,147 of these costs could have been averted if palivizumab had been used.
Conclusions: The net cost to the health care system of using palivizumab, as recommended in the Canadian Paediatric Society guidelines, in study infants in northern Alberta during the 1998/99 RSV season would have been $632,153.
OBJECTIF :: Il est démontré que le palivizumab réduit le taux d’hospitalisation causé par le virus respiratoire syncitial (VRS) chez les prématurés et les nourrissons souffrant d’une maladie pulmonaire chronique. La présente étude visait à établir si le recours au palivizumab pendant la saison 1998–1999 du VRS aurait entraîné des économies par l’attribution aux nourrissons d’un congé d’un hopital d’Edmonton.
MÉTHODOLOGIE :: Une étude rétrospective des hospitalisations au VRS a été exécutée par une communication avec les parents et un examen des listes hospitalières. Le coût net du palivizumab a été établi en comparant le coût d’administration du médicament entre le 1er novembre 1998 et le 1er avril 1999 et le coût de transport des malades et des hospitalisations potentiellement évités.
POPULATION :: Étude de 159 nourrissons ayant obtenu leur congé des hôpitaux d’Edmonton qui ont respecté les critères de la Société canadienne de pédiatrie pour ce qui est de l’administration de palivizumab pendant la saison 1998–1999 du VRS.
RÉSULTATS :: Le coût du recours au palivizumab chez les 159 nourrissons à l’étude aurait été de 753 300 $. Les nourrissons ont été hospitalisés 21 fois en raison du VRS et ont eu besoin de quatre transports médicaux. Le coût estimatif des soins hospitaliers secondaires au VRS s’élevait à 168 888 $ pour ces nourrissons. Si on tient compte d’une efficacité du médicament de 39 % chez les nourrissons souffrant d’une maladie pulmonaire chronique et de 78 % chez ceux nés avant 33 semaines d’âge gestationnel sans maladie pulmonaire chronique, 121 147 $ de ces frais auraient été épargnés si du palivizumab avait été prescrit.
CONCLUSIONS :: Le coût net d’utilisation du palivizumab, tel qu’il est recommandé dans des directives de la Société canadienne de pédiatrie, aurait correspondu à 632 153 $ pour le système de santé du nord de l’Alberta pendant la saison 1998–1999 du VRS.
Keywords: Chronic lung disease; Palivizumab; Prematurity; Respiratory syncytial virus.
Figures
Similar articles
-
Cost-effectiveness of palivizumab in New Zealand.J Paediatr Child Health. 2002 Aug;38(4):352-7. doi: 10.1046/j.1440-1754.2002.00790.x. J Paediatr Child Health. 2002. PMID: 12173995
-
Cost-effectiveness of respiratory syncytial virus prophylaxis among preterm infants.Pediatrics. 1999 Sep;104(3 Pt 1):419-27. doi: 10.1542/peds.104.3.419. Pediatrics. 1999. PMID: 10469764
-
Description of the outcomes of prior authorization of palivizumab for prevention of respiratory syncytial virus infection in a managed care organization.J Manag Care Pharm. 2010 Jan-Feb;16(1):15-22. doi: 10.18553/jmcp.2010.16.1.15. J Manag Care Pharm. 2010. PMID: 20044843 Free PMC article.
-
Impact of the 2014 American Academy of Pediatrics recommendation and of the resulting limited financial coverage by the Italian Medicines Agency for palivizumab prophylaxis on the RSV-associated hospitalizations in preterm infants during the 2016-2017 epidemic season: a systematic review of seven Italian reports.Ital J Pediatr. 2019 Nov 9;45(1):139. doi: 10.1186/s13052-019-0736-5. Ital J Pediatr. 2019. PMID: 31706338 Free PMC article.
-
Updated cost-effectiveness analysis of palivizumab (Synagis) for the prophylaxis of respiratory syncytial virus in infant populations in the UK.J Med Econ. 2020 Dec;23(12):1640-1652. doi: 10.1080/13696998.2020.1836923. Epub 2020 Oct 27. J Med Econ. 2020. PMID: 33107769 Review.
References
-
- The PREVENT Study Group Reduction of respiratory syncytial virus hospitalization among premature infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–9. - PubMed
-
- The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–7. - PubMed
-
- American Academy of Pediatrics Prevention of respiratory syncytial virus infections: Indications for the use of palivizumab and update on the use of RSV-IGIV. Pediatrics. 1998;102:1211–6. - PubMed
-
- Lee SL, Robinson JL. Questions about Palivizumab (Synagis) Pediatrics. 1999;103:535. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
