Cdk4 regulates recruitment of quiescent beta-cells and ductal epithelial progenitors to reconstitute beta-cell mass
- PMID: 20084282
- PMCID: PMC2801612
- DOI: 10.1371/journal.pone.0008653
Cdk4 regulates recruitment of quiescent beta-cells and ductal epithelial progenitors to reconstitute beta-cell mass
Abstract
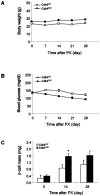
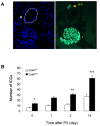
Insulin-producing pancreatic islet beta cells (beta-cells) are destroyed, severely depleted or functionally impaired in diabetes. Therefore, replacing functional beta-cell mass would advance clinical diabetes management. We have previously demonstrated the importance of Cdk4 in regulating beta-cell mass. Cdk4-deficient mice display beta-cell hypoplasia and develop diabetes, whereas beta-cell hyperplasia is observed in mice expressing an active Cdk4R24C kinase. While beta-cell replication appears to be the primary mechanism responsible for beta-cell mass increase, considerable evidence also supports a contribution from the pancreatic ductal epithelium in generation of new beta-cells. Further, while it is believed that majority of beta-cells are in a state of 'dormancy', it is unclear if and to what extent the quiescent cells can be coaxed to participate in the beta-cell regenerative response. Here, we address these queries using a model of partial pancreatectomy (PX) in Cdk4 mutant mice. To investigate the kinetics of the regeneration process precisely, we performed DNA analog-based lineage-tracing studies followed by mathematical modeling. Within a week after PX, we observed considerable proliferation of islet beta-cells and ductal epithelial cells. Interestingly, the mathematical model showed that recruitment of quiescent cells into the active cell cycle promotes beta-cell mass reconstitution in the Cdk4R24C pancreas. Moreover, within 24-48 hours post-PX, ductal epithelial cells expressing the transcription factor Pdx-1 dramatically increased. We also detected insulin-positive cells in the ductal epithelium along with a significant increase of islet-like cell clusters in the Cdk4R24C pancreas. We conclude that Cdk4 not only promotes beta-cell replication, but also facilitates the activation of beta-cell progenitors in the ductal epithelium. In addition, we show that Cdk4 controls beta-cell mass by recruiting quiescent cells to enter the cell cycle. Comparing the contribution of cell proliferation and islet-like clusters to the total increase in insulin-positive cells suggests a hitherto uncharacterized large non-proliferative contribution.
Conflict of interest statement
Figures


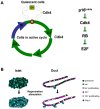
 quiescent (black) and
quiescent (black) and  active (white) cells are subjected to two pulse (CldU and IdU) and one chase periods. The numbers of once- and twice-proliferated cells are represented by
active (white) cells are subjected to two pulse (CldU and IdU) and one chase periods. The numbers of once- and twice-proliferated cells are represented by  and
and  (during the CldU period);
(during the CldU period);  and
and  (during the IdU period); and
(during the IdU period); and  and
and  (during the chase period). The number of purple twice-proliferated (once in CldU and once in IdU) cells are denoted with
(during the chase period). The number of purple twice-proliferated (once in CldU and once in IdU) cells are denoted with  . Note that some labeled cells divide during the chase period and decrease their labeling intensity. They are described with slashed green and red cells in the chase period. (B) Three protocols of the double-labeling experiment with two pulse periods and following chase period.
. Note that some labeled cells divide during the chase period and decrease their labeling intensity. They are described with slashed green and red cells in the chase period. (B) Three protocols of the double-labeling experiment with two pulse periods and following chase period.
 ,
, , and
, and  ) normalized by initial active cell number; proliferation rate of active cells; and ratio of proliferated cells among entire cell population are plotted.
) normalized by initial active cell number; proliferation rate of active cells; and ratio of proliferated cells among entire cell population are plotted.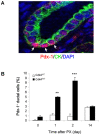
Similar articles
-
Pancreatic islets from cyclin-dependent kinase 4/R24C (Cdk4) knockin mice have significantly increased beta cell mass and are physiologically functional, indicating that Cdk4 is a potential target for pancreatic beta cell mass regeneration in Type 1 diabetes.Diabetologia. 2004 Apr;47(4):686-94. doi: 10.1007/s00125-004-1372-0. Diabetologia. 2004. PMID: 15298346
-
In vivo proliferation of differentiated pancreatic islet beta cells in transgenic mice expressing mutated cyclin-dependent kinase 4.Diabetologia. 2004 Oct;47(10):1819-30. doi: 10.1007/s00125-004-1522-4. Epub 2004 Oct 6. Diabetologia. 2004. PMID: 15480536
-
Impaired beta-cell regeneration after partial pancreatectomy in the adult Goto-Kakizaki rat, a spontaneous model of type II diabetes.Histochem Cell Biol. 2001 Aug;116(2):131-9. doi: 10.1007/s004180100302. Histochem Cell Biol. 2001. PMID: 11685541
-
Can pancreatic duct-derived progenitors be a source of islet regeneration?Biochem Biophys Res Commun. 2009 Jun 12;383(4):383-5. doi: 10.1016/j.bbrc.2009.03.114. Epub 2009 Mar 24. Biochem Biophys Res Commun. 2009. PMID: 19324022 Review.
-
Pancreatic progenitors: The shortest route to restore islet cell mass.Islets. 2011 Nov-Dec;3(6):295-301. doi: 10.4161/isl.3.6.17704. Epub 2011 Nov 1. Islets. 2011. PMID: 21934353 Review.
Cited by
-
Development and regeneration in the endocrine pancreas.ISRN Endocrinol. 2012;2012:640956. doi: 10.5402/2012/640956. Epub 2012 Dec 27. ISRN Endocrinol. 2012. PMID: 23326678 Free PMC article.
-
Geniposide promotes beta-cell regeneration and survival through regulating β-catenin/TCF7L2 pathway.Cell Death Dis. 2015 May 7;6(5):e1746. doi: 10.1038/cddis.2015.107. Cell Death Dis. 2015. PMID: 25950476 Free PMC article.
-
FBX8 promotes metastatic dormancy of colorectal cancer in liver.Cell Death Dis. 2020 Aug 14;11(8):622. doi: 10.1038/s41419-020-02870-7. Cell Death Dis. 2020. PMID: 32796813 Free PMC article.
-
Pancreas β cell regeneration and type 1 diabetes (Review).Exp Ther Med. 2015 Mar;9(3):653-657. doi: 10.3892/etm.2014.2163. Epub 2014 Dec 30. Exp Ther Med. 2015. PMID: 25667609 Free PMC article.
-
The cell cycle as a brake for β-cell regeneration from embryonic stem cells.Stem Cell Res Ther. 2016 Jan 13;7:9. doi: 10.1186/s13287-015-0274-z. Stem Cell Res Ther. 2016. PMID: 26759123 Free PMC article. Review.
References
-
- Hellerstrom C. The life story of the pancreatic B cell. Diabetologia. 1984;26:393–400. - PubMed
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. - PubMed
-
- Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. - PubMed
-
- Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl 3):S16–21. - PubMed
-
- Hellerstrom C, Swenne I. Functional maturation and proliferation of fetal pancreatic beta-cells. Diabetes. 1991;40(Suppl 2):89–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

