Squamous carcinoma cells influence monocyte phenotype and suppress lipopolysaccharide-induced TNF-alpha in monocytes
- PMID: 20084448
- PMCID: PMC2888725
- DOI: 10.1007/s10753-009-9175-6
Squamous carcinoma cells influence monocyte phenotype and suppress lipopolysaccharide-induced TNF-alpha in monocytes
Abstract
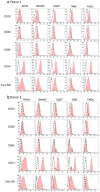
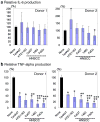
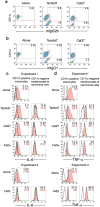
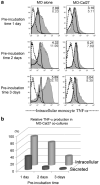
Bacteria and chronic inflammation are present in squamous cell carcinoma of the head and neck (HNSCC), but their roles in the pathogenesis of HNSCC are unclear. Our studies described here revealed that human monocytes co-cultured short term with HNSCC cells were more likely to express CD16, and CD16(+) small mononuclear cells were common in HNSCC specimens. In addition, we identified monocytes as the primary source of LPS-induced IL-6 and TNF-alpha in the monocyte-HNSCC co-cultures. Remarkably, relative to LPS-stimulated monocytes cultured alone, HNSCC cells profoundly suppressed LPS-induced TNF-alpha in monocytes, without compromising IL-6 production. High levels of cytoprotective factors like IL-6 and low levels of TNF-alpha are important for the tumor microenvironment that enables tumor cell survival, affects monocyte differentiation and may contribute to tumor colonization by bacteria. This study provides novel observations that HNSCC cells affect monocyte phenotype and function, which are relevant to the regulation of the HNSCC microenvironment.
Figures




References
-
- Gallo O, Gori AM, Attanasio M, Martini F, Giusti B, Brunelli T, et al. Interleukin-6 and acute-phase proteins in head and neck cancer. European Archives of Oto-Rhino-Laryngology. 1995;252:159–162. - PubMed
-
- Druzgal CH, Chen Z, Yeh NT, Thomas GR, Ondrey FG, Duffey DC, et al. A pilot study of longitudinal serum cytokine and angiogenesis factor levels as markers of therapeutic response and survival in patients with head and neck squamous cell carcinoma. Head and Neck. 2005;27:771–784. - PubMed
-
- Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clinical Cancer Research. 1999;5:1369–1379. - PubMed
-
- Sparano A, Lathers DM, Achille N, Petruzzelli GJ, Young MR. Modulation of Th1 and Th2 cytokine profiles and their association with advanced head and neck squamous cell carcinoma. Otolaryngology - Head and Neck Surgery. 2004;131:573–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

