HPLC-based activity profiling: discovery of piperine as a positive GABA(A) receptor modulator targeting a benzodiazepine-independent binding site
- PMID: 20085307
- PMCID: PMC3196983
- DOI: 10.1021/np900656g
HPLC-based activity profiling: discovery of piperine as a positive GABA(A) receptor modulator targeting a benzodiazepine-independent binding site
Abstract
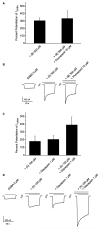
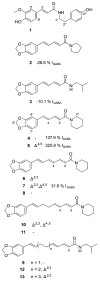
A plant extract library was screened for GABA(A) receptor activity making use of a two-microelectrode voltage clamp assay on Xenopus laevis oocytes. An ethyl acetate extract of black pepper fruits [Piper nigrum L. (Piperaceae) 100 microg/mL] potentiated GABA-induced chloride currents through GABA(A) receptors (composed of alpha(1), beta(2), and gamma(2S) subunits) by 169.1 +/- 2.4%. With the aid of an HPLC-based activity profiling approach, piperine (5) was identified as the main active compound, together with 12 structurally related less active or inactive piperamides (1-4, 6-13). Identification was achieved by on-line high-resolution mass spectrometry and off-line microprobe 1D and 2D NMR spectroscopy, using only milligram amounts of extract. Compound 5 induced a maximum potentiation of the chloride currents by 301.9 +/- 26.5% with an EC(50) of 52.4 +/- 9.4 microM. A comparison of the modulatory activity of 5 and other naturally occurring piperamides enabled insights into structural features critical for GABA(A) receptor modulation. The stimulation of chloride currents through GABA(A) receptors by compound 5 was not antagonized by flumazenil (10 microM). These data show that piperine (5) represents a new scaffold of positive allosteric GABA(A) receptor modulators targeting a benzodiazepine-independent binding site.
Figures



Similar articles
-
GABAA receptor modulation by piperine and a non-TRPV1 activating derivative.Biochem Pharmacol. 2013 Jun 15;85(12):1827-36. doi: 10.1016/j.bcp.2013.04.017. Epub 2013 Apr 25. Biochem Pharmacol. 2013. PMID: 23623790 Free PMC article.
-
Piper nigrum and piperine: an update.Phytother Res. 2013 Aug;27(8):1121-30. doi: 10.1002/ptr.4972. Epub 2013 Apr 29. Phytother Res. 2013. PMID: 23625885 Review.
-
A FLIPR Assay for Discovery of GABAA Receptor Modulators of Natural Origin.Planta Med. 2019 Aug;85(11-12):925-933. doi: 10.1055/a-0921-7602. Epub 2019 May 24. Planta Med. 2019. PMID: 31127604
-
Piperine Congeners as Inhibitors of Vascular Smooth Muscle Cell Proliferation.Planta Med. 2015 Aug;81(12-13):1065-74. doi: 10.1055/s-0035-1546165. Epub 2015 Jul 1. Planta Med. 2015. PMID: 26132851
-
A critical review on the extraction and pharmacotherapeutic activity of piperine.Polim Med. 2022 Jan-Jun;52(1):31-36. doi: 10.17219/pim/145512. Polim Med. 2022. PMID: 35196422 Review.
Cited by
-
Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1-42) rat model of Alzheimer's disease.Behav Brain Funct. 2015 Mar 29;11:13. doi: 10.1186/s12993-015-0059-7. Behav Brain Funct. 2015. PMID: 25880991 Free PMC article.
-
Insights into structure-activity relationship of GABAA receptor modulating coumarins and furanocoumarins.Eur J Pharmacol. 2011 Oct 1;668(1-2):57-64. doi: 10.1016/j.ejphar.2011.06.034. Epub 2011 Jul 5. Eur J Pharmacol. 2011. PMID: 21749864 Free PMC article.
-
Identification of GABA A receptor modulators in Kadsura longipedunculata and assignment of absolute configurations by quantum-chemical ECD calculations.Phytochemistry. 2011 Dec;72(18):2385-95. doi: 10.1016/j.phytochem.2011.08.014. Epub 2011 Aug 31. Phytochemistry. 2011. PMID: 21889177 Free PMC article.
-
GABAA receptor modulation by piperine and a non-TRPV1 activating derivative.Biochem Pharmacol. 2013 Jun 15;85(12):1827-36. doi: 10.1016/j.bcp.2013.04.017. Epub 2013 Apr 25. Biochem Pharmacol. 2013. PMID: 23623790 Free PMC article.
-
HPLC-based activity profiling for GABAA receptor modulators from the traditional Chinese herbal drug Kushen (Sophora flavescens root).Mol Divers. 2011 May;15(2):361-72. doi: 10.1007/s11030-010-9297-7. Epub 2011 Jan 5. Mol Divers. 2011. PMID: 21207144 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

