Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry
- PMID: 20085328
- PMCID: PMC2836843
- DOI: 10.1021/jm9015729
Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry
Abstract
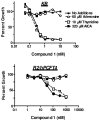
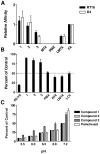
2-Amino-4-oxo-6-substituted pyrrolo[2,3-d]pyrimidines with a thienoyl side chain and four to six carbon bridge lengths (compounds 1-3) were synthesized as substrates for folate receptors (FRs) and the proton-coupled folate transporter (PCFT). Conversion of acetylene carboxylic acids to alpha-bromomethylketones and condensation with 2,4-diamino-6-hydroxypyrimidine afforded the 6-substituted pyrrolo[2,3-d]pyrimidines. Sonogashira coupling with (S)-2-[(5-bromo-thiophene-2-carbonyl)-amino]-pentanedioic acid diethyl ester, followed by hydrogenation and saponification, afforded 1-3. Compounds 1 and 2 potently inhibited KB and IGROV1 human tumor cells that express FR alpha, reduced folate carrier (RFC), and PCFT. The analogs were selective for FR and PCFT over RFC. Glycinamide ribonucleotide formyltransferase was the principal cellular target. In SCID mice with KB tumors, 1 was highly active against both early (3.5 log kill, 1/5 cures) and advanced (3.7 log kill, 4/5 complete remissions) stage tumors. Our results demonstrate potent in vitro and in vivo antitumor activity for 1 due to selective transport by FRs and PCFT over RFC.
Figures
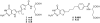




Similar articles
-
Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review.
-
Synthesis and biological activity of a novel series of 6-substituted thieno[2,3-d]pyrimidine antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors over the reduced folate carrier and proton-coupled folate transporter for cellular entry.J Med Chem. 2009 May 14;52(9):2940-51. doi: 10.1021/jm8011323. J Med Chem. 2009. PMID: 19371039 Free PMC article.
-
Synthesis, biological, and antitumor activity of a highly potent 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitor with proton-coupled folate transporter and folate receptor selectivity over the reduced folate carrier that inhibits β-glycinamide ribonucleotide formyltransferase.J Med Chem. 2011 Oct 27;54(20):7150-64. doi: 10.1021/jm200739e. Epub 2011 Sep 22. J Med Chem. 2011. PMID: 21879757 Free PMC article.
-
6-Substituted Pyrrolo[2,3-d]pyrimidine Thienoyl Regioisomers as Targeted Antifolates for Folate Receptor α and the Proton-Coupled Folate Transporter in Human Tumors.J Med Chem. 2015 Sep 10;58(17):6938-59. doi: 10.1021/acs.jmedchem.5b00801. Epub 2015 Aug 28. J Med Chem. 2015. PMID: 26317331 Free PMC article.
-
The promise and challenges of exploiting the proton-coupled folate transporter for selective therapeutic targeting of cancer.Cancer Chemother Pharmacol. 2018 Jan;81(1):1-15. doi: 10.1007/s00280-017-3473-8. Epub 2017 Nov 10. Cancer Chemother Pharmacol. 2018. PMID: 29127457 Free PMC article. Review.
Cited by
-
Structural determinants of human proton-coupled folate transporter oligomerization: role of GXXXG motifs and identification of oligomeric interfaces at transmembrane domains 3 and 6.Biochem J. 2015 Jul 1;469(1):33-44. doi: 10.1042/BJ20150169. Epub 2015 Apr 16. Biochem J. 2015. PMID: 25877470 Free PMC article.
-
Functional and mechanistic roles of the human proton-coupled folate transporter transmembrane domain 6-7 linker.Biochem J. 2016 Oct 15;473(20):3545-3562. doi: 10.1042/BCJ20160399. Epub 2016 Aug 11. Biochem J. 2016. PMID: 27514717 Free PMC article.
-
Tumor Targeting with Novel Pyridyl 6-Substituted Pyrrolo[2,3- d]Pyrimidine Antifolates via Cellular Uptake by Folate Receptor α and the Proton-Coupled Folate Transporter and Inhibition of De Novo Purine Nucleotide Biosynthesis.J Med Chem. 2018 Mar 8;61(5):2027-2040. doi: 10.1021/acs.jmedchem.7b01708. Epub 2018 Feb 21. J Med Chem. 2018. PMID: 29425443 Free PMC article.
-
Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review.
-
Biology and therapeutic applications of the proton-coupled folate transporter.Expert Opin Drug Metab Toxicol. 2022 Oct;18(10):695-706. doi: 10.1080/17425255.2022.2136071. Epub 2022 Oct 20. Expert Opin Drug Metab Toxicol. 2022. PMID: 36239195 Free PMC article.
References
-
- Monahan BP, Allegra CJ. Antifolates. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. 4th Edison Lippincott-Raven; Philadelphia: 2001. pp. 109–148.
-
- Hazarika M, White RM, Johnson JR, Pazdur R. FDA Drug Approval Summaries: Pemetrexed (Alimta) Oncologist. 2004;9:482–488. - PubMed
-
- Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D. Phase III Study Comparing Cisplatin Plus Gemcitabine With Cisplatin Plus Pemetrexed in Chemotherapy-Naive Patients With Advanced-Stage Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2008;26:3543–3551. - PubMed
-
- Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, Laack E, Wu YL, Bover I, Begbie S, Tzekova V, Cucevic B, Pereira JR, Yang SH, Madhavan J, Sugarman KP, Peterson P, John WJ, Krejcy K, Belani CP. Maintenance Pemetrexed Plus Best Supportive Care Versus Placebo Plus Best Supportive Care for Non-Small-Cell Lung Cancer: A Randomised, Double-Blind, Phase 3 Study. Lancet. 2009;374:1432–1440. - PubMed
-
- Chu E, Callender MA, Farrell MP, Schmitz JC. Thymidylate Synthase Inhibitors as Anticancer Agents: From Bench to Bedside. Cancer Chemother. Pharmacol. 2003;52(Suppl 1):S80–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

