Substrate specificity combined with stereopromiscuity in glutathione transferase A4-4-dependent metabolism of 4-hydroxynonenal
- PMID: 20085333
- PMCID: PMC2829664
- DOI: 10.1021/bi902038u
Substrate specificity combined with stereopromiscuity in glutathione transferase A4-4-dependent metabolism of 4-hydroxynonenal
Abstract
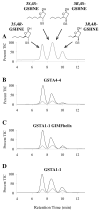
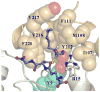
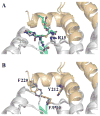
Conjugation to glutathione (GSH) by glutathione transferase A4-4 (GSTA4-4) is a major route of elimination for the lipid peroxidation product 4-hydroxynonenal (HNE), a toxic compound that contributes to numerous diseases. Both enantiomers of HNE are presumed to be toxic, and GSTA4-4 has negligible stereoselectivity toward them, despite its high catalytic chemospecificity for alkenals. In contrast to the highly flexible, and substrate promiscuous, GSTA1-1 isoform that has poor catalytic efficiency with HNE, GSTA4-4 has been postulated to be a rigid template that is preorganized for HNE metabolism. However, the combination of high substrate chemoselectivity and low substrate stereoselectivity is intriguing. The mechanism by which GSTA4-4 achieves this combination is important, because it must metabolize both enantiomers of HNE to efficiently detoxify the biologically formed mixture. The crystal structures of GSTA4-4 and an engineered variant of GSTA1-1 with high catalytic efficiency toward HNE, cocrystallized with a GSH-HNE conjugate analogue, demonstrate that GSTA4-4 undergoes no enantiospecific induced fit; instead, the active site residue Arg15 is ideally located to interact with the 4-hydroxyl group of either HNE enantiomer. The results reveal an evolutionary strategy for achieving biologically useful stereopromiscuity toward a toxic racemate, concomitant with high catalytic efficiency and substrate specificity toward an endogenously formed toxin.
Figures
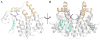
References
-
- Ansari NH, Wang L, Srivastava SK. Role of lipid aldehydes in cataractogenesis: 4-hydroxynonenal-induced cataract. Biochem Mol Med. 1996;58:25–30. - PubMed
-
- Grimsrud PA, Picklo MJ, Sr, Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6:624–637. - PubMed
-
- Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–1789. - PubMed
-
- Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003;24:293–303. - PubMed
-
- Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23:281–305. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

