Long-term efficacy and safety of Raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trials
- PMID: 20085491
- PMCID: PMC6076431
- DOI: 10.1086/650002
Long-term efficacy and safety of Raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 Phase III trials
Abstract
BENCHMRK-1 and -2 are ongoing double-blind phase III studies of raltegravir in patients experiencing failure of antiretroviral therapy with triple-class drug-resistant human immunodeficiency virus infection. At week 96 (combined data), raltegravir (400 mg twice daily) plus optimized background therapy was generally well tolerated, with superior and durable antiretroviral and immunological efficacy, compared with optimized background therapy alone.
Conflict of interest statement
Figures
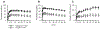


References
-
- Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 2000; 287:646–650. - PubMed
-
- Miller M, Witmer M, Stillmock K, et al. Biochemical and antiviral activity of MK-0518, a potent HIV integrase inhibitor. In: Program and abstracts of the 16th International AIDS Conference (Toronto, Canada). 2006. Abstract THAA0302.
-
- Markowitz M, Nguyen B-Y, Gotuzzo E, et al.; Protocol 004 Part II Study Team. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naive patients with HIV-1 infection. J Acquir Immune Defic Syndr 2009; 52:350–356. - PubMed
-
- Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med 2008; 359:339–354. - PubMed

