A new class of dual-targeted antivirals: monophosphorylated acyclovir prodrug derivatives suppress both human immunodeficiency virus type 1 and herpes simplex virus type 2
- PMID: 20085496
- PMCID: PMC2811219
- DOI: 10.1086/650343
A new class of dual-targeted antivirals: monophosphorylated acyclovir prodrug derivatives suppress both human immunodeficiency virus type 1 and herpes simplex virus type 2
Abstract
Background: Human immunodeficiency virus type 1 (HIV-1) and herpes simplex virus type 2 (HSV-2) are responsible for 2 intersecting epidemics in which the disease caused by 1 virus facilitates the transmission of and pathogenesis by the other. Therefore, suppression of one virus infection will affect the other. Acyclovir, a common antiherpetic drug, was shown to directly suppress both viruses in coinfected tissues. However, both antiviral activities of acyclovir are dependent on phosphorylation by the nucleoside kinase activity of coinfecting human herpesviruses.
Methods: We developed acyclovir ProTides, monophosphorylated acyclovir with the phosphate group masked by lipophilic groups to allow efficient cellular uptake, and investigated their antiviral potential in cell lines and in human tissues ex vivo.
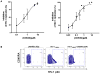
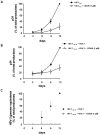
Results: Acyclovir ProTides suppressed both HIV-1 and HSV-2 at median effective concentrations in the submicromolar range in ex vivo lymphoid and cervicovaginal human tissues and at 3-12 micromol/L in CD4(+) T cells. Acyclovir ProTides retained activity against acyclovir-resistant HSV-2.
Conclusions: Acyclovir ProTides represent a new class of antivirals that suppress both HIV-1 and HSV-2 by directly and independently blocking the key replicative enzymes of both viruses. Further optimization of such compounds may lead to double-targeted antivirals that can prevent viral transmission and treat the 2 synergistic diseases caused by HIV-1 and HSV-2. To our knowledge, the acyclovir ProTides described here represent the first example of acyclic nucleoside monophosphate prodrugs being active against HIV-1.
Conflict of interest statement
The authors do not have a commercial or other association that might pose a conflict of interest.
Figures
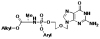
Similar articles
-
Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus.N Engl J Med. 2007 Feb 22;356(8):790-9. doi: 10.1056/NEJMoa062607. N Engl J Med. 2007. PMID: 17314338 Clinical Trial.
-
Use of acyclovir for suppression of human immunodeficiency virus infection is not associated with genotypic evidence of herpes simplex virus type 2 resistance to acyclovir: analysis of specimens from three phase III trials.J Clin Microbiol. 2010 Oct;48(10):3496-503. doi: 10.1128/JCM.01263-10. Epub 2010 Aug 11. J Clin Microbiol. 2010. PMID: 20702659 Free PMC article. Clinical Trial.
-
Surgical excision for recurrent herpes simplex virus 2 (HSV-2) anogenital infection in a patient with human immunodeficiency virus (HIV).Infection. 2017 Oct;45(5):705-707. doi: 10.1007/s15010-017-1027-y. Epub 2017 May 15. Infection. 2017. PMID: 28508238 Free PMC article.
-
Infectious co-factors in HIV-1 transmission herpes simplex virus type-2 and HIV-1: new insights and interventions.Curr HIV Res. 2012 Apr;10(3):228-37. doi: 10.2174/157016212800618156. Curr HIV Res. 2012. PMID: 22384842 Free PMC article. Review.
-
Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir.J Infect Dis. 2002 Oct 15;186 Suppl 1:S40-6. doi: 10.1086/342966. J Infect Dis. 2002. PMID: 12353186 Review.
Cited by
-
Update on emerging antivirals for the management of herpes simplex virus infections: a patenting perspective.Recent Pat Antiinfect Drug Discov. 2013 Apr;8(1):55-67. doi: 10.2174/1574891x11308010011. Recent Pat Antiinfect Drug Discov. 2013. PMID: 23331181 Free PMC article. Review.
-
Herpes simplex virus-induced epithelial damage and susceptibility to human immunodeficiency virus type 1 infection in human cervical organ culture.PLoS One. 2011;6(7):e22638. doi: 10.1371/journal.pone.0022638. Epub 2011 Jul 27. PLoS One. 2011. PMID: 21818356 Free PMC article.
-
Prodrugs of phosphonates and phosphates: crossing the membrane barrier.Top Curr Chem. 2015;360:115-60. doi: 10.1007/128_2014_561. Top Curr Chem. 2015. PMID: 25391982 Free PMC article. Review.
-
Exploiting the anti-HIV-1 activity of acyclovir: suppression of primary and drug-resistant HIV isolates and potentiation of the activity by ribavirin.Antimicrob Agents Chemother. 2012 May;56(5):2604-11. doi: 10.1128/AAC.05986-11. Epub 2012 Feb 6. Antimicrob Agents Chemother. 2012. PMID: 22314523 Free PMC article.
-
Dual-targeted anti-CMV/anti-HIV-1 heterodimers.Biochimie. 2021 Oct;189:169-180. doi: 10.1016/j.biochi.2021.06.011. Epub 2021 Jun 29. Biochimie. 2021. PMID: 34197866 Free PMC article.
References
-
- Cohen MS. HIV and sexually transmitted diseases: lethal synergy. Top HIV Med. 2004;12:104–7. - PubMed
-
- Corey L. Synergistic copathogens--HIV-1 and HSV-2. N Engl J Med. 2007;356:854–6. - PubMed
-
- Wenner M. Virology: the battle within. Nature. 2008;451:388–9. - PubMed
-
- Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infect Dis. 2008;8:490–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

