Stimulation of GLUT4 (glucose transporter isoform 4) storage vesicle formation by sphingolipid depletion
- PMID: 20085539
- PMCID: PMC2838997
- DOI: 10.1042/BJ20091529
Stimulation of GLUT4 (glucose transporter isoform 4) storage vesicle formation by sphingolipid depletion
Abstract
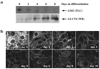
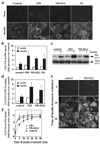
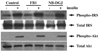
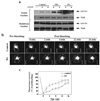
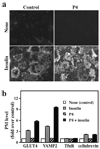
Insulin stimulates glucose transport in fat and skeletal muscle cells primarily by inducing the translocation of GLUT4 (glucose transporter isoform 4) to the PM (plasma membrane) from specialized GSVs (GLUT4 storage vesicles). Glycosphingolipids are components of membrane microdomains and are involved in insulin-regulated glucose transport. Cellular glycosphingolipids decrease during adipocyte differentiation and have been suggested to be involved in adipocyte function. In the present study, we investigated the role of glycosphingolipids in regulating GLUT4 translocation. We decreased glycosphingolipids in 3T3-L1 adipocytes using glycosphingolipid synthesis inhibitors and investigated the effects on GLUT4 translocation using immunocytochemistry, preparation of PM sheets, isolation of GSVs and FRAP (fluorescence recovery after photobleaching) of GLUT4-GFP (green fluorescent protein) in intracellular structures. Glycosphingolipids were located in endosomal vesicles in pre-adipocytes and redistributed to the PM with decreased expression at day 2 after initiation of differentiation. In fully differentiated adipocytes, depletion of glycosphingolipids dramatically accelerated insulin-stimulated GLUT4 translocation. Although insulin-induced phosphorylation of IRS (insulin receptor substrate) and Akt remained intact in glycosphingolipid-depleted cells, both in vitro budding of GLUT4 vesicles and FRAP of GLUT4-GFP on GSVs were stimulated. Glycosphingolipid depletion also enhanced the insulin-induced translocation of VAMP2 (vesicle-associated membrane protein 2), but not the transferrin receptor or cellubrevin, indicating that the effect of glycosphingolipids was specific to VAMP2-positive GSVs. Our results strongly suggest that decreasing glycosphingolipid levels promotes the formation of GSVs and, thus, GLUT4 translocation. These studies provide a mechanistic basis for recent studies showing that inhibition of glycosphingolipid synthesis improves glycaemic control and enhances insulin sensitivity in animal models of Type 2 diabetes.
Figures
Similar articles
-
Myosin IIA participates in docking of Glut4 storage vesicles with the plasma membrane in 3T3-L1 adipocytes.Biochem Biophys Res Commun. 2010 Jan 1;391(1):995-9. doi: 10.1016/j.bbrc.2009.12.004. Epub 2009 Dec 5. Biochem Biophys Res Commun. 2010. PMID: 19968963
-
Cellubrevin is a resident protein of insulin-sensitive GLUT4 glucose transporter vesicles in 3T3-L1 adipocytes.J Biol Chem. 1995 Apr 7;270(14):8233-40. doi: 10.1074/jbc.270.14.8233. J Biol Chem. 1995. PMID: 7713930
-
Insulin-regulated Glut4 translocation: membrane protein trafficking with six distinctive steps.J Biol Chem. 2014 Jun 20;289(25):17280-98. doi: 10.1074/jbc.M114.555714. Epub 2014 Apr 28. J Biol Chem. 2014. PMID: 24778187 Free PMC article.
-
Role of SNARE's in the GLUT4 translocation response to insulin in adipose cells and muscle.J Basic Clin Physiol Pharmacol. 1998;9(2-4):153-65. doi: 10.1515/jbcpp.1998.9.2-4.153. J Basic Clin Physiol Pharmacol. 1998. PMID: 10212832 Review.
-
Posttranslational modifications of GLUT4 affect its subcellular localization and translocation.Int J Mol Sci. 2013 May 10;14(5):9963-78. doi: 10.3390/ijms14059963. Int J Mol Sci. 2013. PMID: 23665900 Free PMC article. Review.
Cited by
-
CHC22 clathrin recruitment to the early secretory pathway requires two-site interaction with SNX5 and p115.EMBO J. 2024 Oct;43(19):4298-4323. doi: 10.1038/s44318-024-00198-y. Epub 2024 Aug 19. EMBO J. 2024. PMID: 39160272 Free PMC article.
-
Glycosphingolipids mediate pneumocystis cell wall β-glucan activation of the IL-23/IL-17 axis in human dendritic cells.Am J Respir Cell Mol Biol. 2012 Jul;47(1):50-9. doi: 10.1165/rcmb.2011-0159OC. Epub 2012 Feb 16. Am J Respir Cell Mol Biol. 2012. PMID: 22343219 Free PMC article.
-
Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle.Arch Surg. 2011 May;146(5):556-64. doi: 10.1001/archsurg.2011.100. Arch Surg. 2011. PMID: 21739664 Free PMC article.
-
Hijacking the endocytic machinery by microbial pathogens.Protoplasma. 2010 Aug;244(1-4):75-90. doi: 10.1007/s00709-010-0164-2. Epub 2010 Jun 25. Protoplasma. 2010. PMID: 20574860 Review.
References
-
- Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. - PubMed
-
- Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. - PubMed
-
- Xu Z, Kandror KV. Translocation of small preformed vesicles is responsible for the insulin activation of glucose transport in adipose cells. Evidence from the in vitro reconstitution assay. J. Biol. Chem. 2002;277:47972–47975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

