Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells
- PMID: 20085644
- PMCID: PMC2820018
- DOI: 10.1186/1476-4598-9-9
Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells
Abstract
Background: Myeloid cell leukemia-1 (Mcl-1) is a member of the Bcl-2 family, which inhibits cell apoptosis by sequestering pro-apoptotic proteins Bim and Bid. Mcl-1 overexpression has been associated with progression in leukemia and some solid tumors including prostate cancer (PCa). However, the regulatory mechanism for Mcl-1 expression in PCa cells remains elusive.
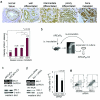
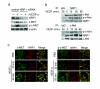
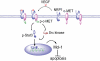
Results: Immunohistochemical analyses revealed that Mcl-1 expression was elevated in PCa specimens with high Gleason grades and further significantly increased in bone metastasis, suggesting a pivotal role of Mcl-1 in PCa metastasis. We further found that vascular endothelial growth factor (VEGF) is a novel regulator of Mcl-1 expression in PCa cells. Inhibition of endogenous Mcl-1 induced apoptosis, indicating that Mcl-1 is an important survival factor in PCa cells. Neuropilin-1 (NRP1), the "co-receptor" for VEGF165 isoform, was found to be highly expressed in PCa cells, and indispensible in the regulation of Mcl-1. Intriguingly, VEGF165 promoted physical interaction between NRP1 and hepatocyte growth factor (HGF) receptor c-MET, and facilitated c-MET phosphorylation via a NRP1-dependent mechanism. VEGF165 induction of Mcl-1 may involve rapid activation of Src kinases and signal transducers and activators of transcription 3 (Stat3). Importantly, NRP1 overexpression and c-MET activation were positively associated with progression and bone metastasis in human PCa specimens and xenograft tissues.
Conclusions: This study demonstrated that Mcl-1 overexpression is associated with PCa bone metastasis. Activation of VEGF165-NRP1-c-MET signaling could confer PCa cells survival advantages by up-regulating Mcl-1, contributing to PCa progression.
Figures





Similar articles
-
Neuropilin-1 promotes human glioma progression through potentiating the activity of the HGF/SF autocrine pathway.Oncogene. 2007 Aug 16;26(38):5577-86. doi: 10.1038/sj.onc.1210348. Epub 2007 Mar 19. Oncogene. 2007. PMID: 17369861 Free PMC article.
-
Evodiamine Mitigates Cellular Growth and Promotes Apoptosis by Targeting the c-Met Pathway in Prostate Cancer Cells.Molecules. 2020 Mar 13;25(6):1320. doi: 10.3390/molecules25061320. Molecules. 2020. PMID: 32183146 Free PMC article.
-
Sabutoclax, a Mcl-1 antagonist, inhibits tumorigenesis in transgenic mouse and human xenograft models of prostate cancer.Neoplasia. 2012 Jul;14(7):656-65. doi: 10.1593/neo.12640. Neoplasia. 2012. PMID: 22904682 Free PMC article.
-
How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns.Biochem Soc Trans. 2011 Dec;39(6):1583-91. doi: 10.1042/BST20110697. Biochem Soc Trans. 2011. PMID: 22103491 Review.
-
Neuropilin-1 enforces extracellular matrix signalling via ABL1 to promote angiogenesis.Biochem Soc Trans. 2014 Oct;42(5):1429-34. doi: 10.1042/BST20140141. Biochem Soc Trans. 2014. PMID: 25233427 Review.
Cited by
-
Signaling Pathways That Control Apoptosis in Prostate Cancer.Cancers (Basel). 2021 Feb 24;13(5):937. doi: 10.3390/cancers13050937. Cancers (Basel). 2021. PMID: 33668112 Free PMC article. Review.
-
MET inhibitors in combination with other therapies in non-small cell lung cancer.Transl Lung Cancer Res. 2012 Dec;1(4):238-53. doi: 10.3978/j.issn.2218-6751.2012.10.08. Transl Lung Cancer Res. 2012. PMID: 25806189 Free PMC article. Review.
-
Dual Targeting of Neuropilin-1 and Glucose Transporter for Efficient Fluorescence Imaging of Cancer.Mol Imaging Biol. 2025 Apr;27(2):250-259. doi: 10.1007/s11307-025-01993-7. Epub 2025 Mar 6. Mol Imaging Biol. 2025. PMID: 40048021 Free PMC article.
-
Keratin 13 expression reprograms bone and brain metastases of human prostate cancer cells.Oncotarget. 2016 Dec 20;7(51):84645-84657. doi: 10.18632/oncotarget.13175. Oncotarget. 2016. PMID: 27835867 Free PMC article.
-
The best of both worlds - managing the cancer, saving the bone.Nat Rev Endocrinol. 2016 Jan;12(1):29-42. doi: 10.1038/nrendo.2015.185. Epub 2015 Oct 27. Nat Rev Endocrinol. 2016. PMID: 26503674 Free PMC article. Review.
References
-
- Kitada S, Andersen J, Akar S, Zapata JM, Takayama S, Krajewski S, Wang HG, Zhang X, Bullrich F, Croce CM, Rai K, Hines J, Reed JC. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998;91:3379–3389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

