Factors affecting fungus-induced larval mortality in Anopheles gambiae and Anopheles stephensi
- PMID: 20085659
- PMCID: PMC2817704
- DOI: 10.1186/1475-2875-9-22
Factors affecting fungus-induced larval mortality in Anopheles gambiae and Anopheles stephensi
Abstract
Background: Entomopathogenic fungi have shown great potential for the control of adult malaria vectors. However, their ability to control aquatic stages of anopheline vectors remains largely unexplored. Therefore, how larval characteristics (Anopheles species, age and larval density), fungus (species and concentration) and environmental effects (exposure duration and food availability) influence larval mortality caused by fungus, was studied.
Methods: Laboratory bioassays were performed on the larval stages of Anopheles gambiae and Anopheles stephensi with spores of two fungus species, Metarhizium anisopliae and Beauveria bassiana. For various larval and fungal characteristics and environmental effects the time to death was determined and survival curves established. These curves were compared by Kaplan Meier and Cox regression analyses.
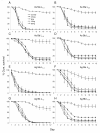
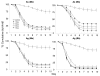
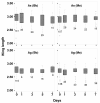
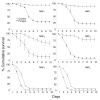
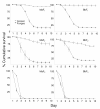
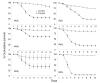
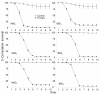
Results: Beauveria bassiana and Metarhizium anisopliae caused high mortality of An. gambiae and An. stephensi larvae. However, Beauveria bassiana was less effective (Hazard ratio (HR) <1) compared to Metarhizium anisopliae. Anopheles stephensi and An. gambiae were equally susceptible to each fungus. Older larvae were less likely to die than young larvae (HR < 1). The effect of increase in fungus concentration on larval mortality was influenced by spore clumping. One day exposure to fungal spores was found to be equally effective as seven days exposure. In different exposure time treatments 0 - 4.9% of the total larvae, exposed to fungus, showed infection at either the pupal or adult stage. Mortality rate increased with increasing larval density and amount of available food.
Conclusions: This study shows that both fungus species have potential to kill mosquitoes in the larval stage, and that mortality rate depends on fungus species itself, larval stage targeted, larval density and amount of nutrients available to the larvae. Increasing the concentration of fungal spores or reducing the exposure time to spores did not show a proportional increase and decrease in mortality rate, respectively, because the spores clumped together. As a result spores did not provide uniform coverage over space and time. It is, therefore, necessary to develop a formulation that allows the spores to spread over the water surface. Apart from formulation appropriate delivery methods are also necessary to avoid exposing non-target organisms to fungus.
Figures
Similar articles
-
Anopheles gambiae pathogen susceptibility: the intersection of genetics, immunity and ecology.Curr Opin Microbiol. 2012 Jun;15(3):285-91. doi: 10.1016/j.mib.2012.04.001. Epub 2012 Apr 24. Curr Opin Microbiol. 2012. PMID: 22538050 Free PMC article. Review.
-
Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae.Parasit Vectors. 2011 Feb 22;4:23. doi: 10.1186/1756-3305-4-23. Parasit Vectors. 2011. PMID: 21342492 Free PMC article.
-
Pyrethroid resistance in Anopheles gambiae leads to increased susceptibility to the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana.Malar J. 2010 Jun 16;9:168. doi: 10.1186/1475-2875-9-168. Malar J. 2010. PMID: 20553597 Free PMC article.
-
Fitness consequences of larval exposure to Beauveria bassiana on adults of the malaria vector Anopheles stephensi.J Invertebr Pathol. 2014 Jun;119:19-24. doi: 10.1016/j.jip.2014.03.003. Epub 2014 Mar 30. J Invertebr Pathol. 2014. PMID: 24694552
-
Direct and indirect effects of predation and parasitism on the Anopheles gambiae mosquito.Parasit Vectors. 2020 Jan 30;13(1):43. doi: 10.1186/s13071-020-3915-8. Parasit Vectors. 2020. PMID: 32000840 Free PMC article. Review.
Cited by
-
The Potential of a New Beauveria bassiana Isolate for Mosquito Larval Control.J Med Entomol. 2023 Jan 12;60(1):131-147. doi: 10.1093/jme/tjac179. J Med Entomol. 2023. PMID: 36633608 Free PMC article.
-
Mode of Infection of Metarhizium spp. Fungus and Their Potential as Biological Control Agents.J Fungi (Basel). 2017 Jun 7;3(2):30. doi: 10.3390/jof3020030. J Fungi (Basel). 2017. PMID: 29371548 Free PMC article. Review.
-
Anopheles gambiae pathogen susceptibility: the intersection of genetics, immunity and ecology.Curr Opin Microbiol. 2012 Jun;15(3):285-91. doi: 10.1016/j.mib.2012.04.001. Epub 2012 Apr 24. Curr Opin Microbiol. 2012. PMID: 22538050 Free PMC article. Review.
-
Genetics and immunity of Anopheles response to the entomopathogenic fungus Metarhizium anisopliae overlap with immunity to Plasmodium.Sci Rep. 2022 Apr 15;12(1):6315. doi: 10.1038/s41598-022-10190-3. Sci Rep. 2022. PMID: 35428783 Free PMC article.
-
Fungal biological control agents for integrated management of Culicoides spp. (Diptera: Ceratopogonidae) of livestock.Vet World. 2015 Feb;8(2):156-63. doi: 10.14202/vetworld.2015.156-163. Epub 2015 Feb 10. Vet World. 2015. PMID: 27047065 Free PMC article.
References
-
- Gu W, Utzinger J, Novak RJ. Habitat-based larval interventions: A new perspective for malaria control. Am J Trop Med Hyg. 2008;78:2–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

