Nonprocessive motor dynamics at the microtubule membrane tube interface
- PMID: 20085722
- PMCID: PMC2800980
- DOI: 10.1016/j.bpj.2009.09.058
Nonprocessive motor dynamics at the microtubule membrane tube interface
Abstract
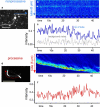
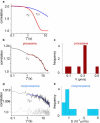
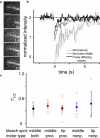
Key cellular processes such as cell division, membrane compartmentalization, and intracellular transport rely on motor proteins. Motors have been studied in detail on the single motor level such that information on their step size, stall force, average run length, and processivity are well known. However, in vivo, motors often work together, so that the question of their collective coordination has raised great interest. Here, we specifically attach motors to giant vesicles and examine collective motor dynamics during membrane tube formation. Image correlation spectroscopy reveals directed motion as processive motors walk at typical speeds (< or = 500 nm/s) along an underlying microtubule and accumulate at the tip of the growing membrane tube. In contrast, nonprocessive motors exhibit purely diffusive behavior, decorating the entire length of a microtubule lattice with diffusion constants at least 1000 times smaller than a freely-diffusing lipid-motor complex in a lipid bilayer (1 microm(2)/s); fluorescence recovery after photobleaching experiments confirm the presence of the slower-moving motor population at the microtubule-membrane tube interface. We suggest that nonprocessive motors dynamically bind and unbind to maintain a continuous interaction with the microtubule. This dynamic and continuous interaction is likely necessary for nonprocessive motors to mediate bidirectional membrane tube dynamics reported previously.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Simulation studies of self-organization of microtubules and molecular motors.Phys Rev E Stat Nonlin Soft Matter Phys. 2008 May;77(5 Pt 1):051905. doi: 10.1103/PhysRevE.77.051905. Epub 2008 May 8. Phys Rev E Stat Nonlin Soft Matter Phys. 2008. PMID: 18643100
-
Bidirectional membrane tube dynamics driven by nonprocessive motors.Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):7993-7. doi: 10.1073/pnas.0709677105. Epub 2008 Mar 10. Proc Natl Acad Sci U S A. 2008. PMID: 18332438 Free PMC article.
-
Cooperative protofilament switching emerges from inter-motor interference in multiple-motor transport.Sci Rep. 2014 Dec 1;4:7255. doi: 10.1038/srep07255. Sci Rep. 2014. PMID: 25434968 Free PMC article.
-
Unconventional functions of microtubule motors.Arch Biochem Biophys. 2012 Apr 1;520(1):17-29. doi: 10.1016/j.abb.2011.12.029. Epub 2012 Jan 28. Arch Biochem Biophys. 2012. PMID: 22306515 Free PMC article. Review.
-
Organisation and structure of microtubules and microtubule-motor protein complexes.Eur Biophys J. 1998;27(5):446-54. doi: 10.1007/s002490050155. Eur Biophys J. 1998. PMID: 9760726 Review.
Cited by
-
Formation of helical membrane tubes around microtubules by single-headed kinesin KIF1A.Nat Commun. 2015 Aug 13;6:8025. doi: 10.1038/ncomms9025. Nat Commun. 2015. PMID: 26268542 Free PMC article.
-
Motor coupling through lipid membranes enhances transport velocities for ensembles of myosin Va.Proc Natl Acad Sci U S A. 2014 Sep 23;111(38):E3986-95. doi: 10.1073/pnas.1406535111. Epub 2014 Sep 8. Proc Natl Acad Sci U S A. 2014. PMID: 25201964 Free PMC article.
-
Modelling cytoskeletal transport by clusters of non-processive molecular motors with limited binding sites.R Soc Open Sci. 2020 Aug 5;7(8):200527. doi: 10.1098/rsos.200527. eCollection 2020 Aug. R Soc Open Sci. 2020. PMID: 32968517 Free PMC article.
-
Kinesin recycling in stationary membrane tubes.Biophys J. 2010 Sep 22;99(6):1835-41. doi: 10.1016/j.bpj.2010.06.071. Biophys J. 2010. PMID: 20858428 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

