Water and backbone dynamics in a hydrated protein
- PMID: 20085726
- PMCID: PMC2800973
- DOI: 10.1016/j.bpj.2009.09.054
Water and backbone dynamics in a hydrated protein
Abstract
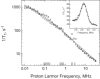
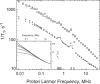
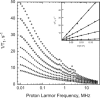
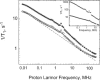
Rotational immobilization of proteins permits characterization of the internal peptide and water molecule dynamics by magnetic relaxation dispersion spectroscopy. Using different experimental approaches, we have extended measurements of the magnetic field dependence of the proton-spin-lattice-relaxation rate by one decade from 0.01 to 300 MHz for (1)H and showed that the underlying dynamics driving the protein (1)H spin-lattice relaxation is preserved over 4.5 decades in frequency. This extension is critical to understanding the role of (1)H(2)O in the total proton-spin-relaxation process. The fact that the protein-proton-relaxation-dispersion profile is a power law in frequency with constant coefficient and exponent over nearly 5 decades indicates that the characteristics of the native protein structural fluctuations that cause proton nuclear spin-lattice relaxation are remarkably constant over this wide frequency and length-scale interval. Comparison of protein-proton-spin-lattice-relaxation rate constants in protein gels equilibrated with (2)H(2)O rather than (1)H(2)O shows that water protons make an important contribution to the total spin-lattice relaxation in the middle of this frequency range for hydrated proteins because of water molecule dynamics in the time range of tens of ns. This water contribution is with the motion of relatively rare, long-lived, and perhaps buried water molecules constrained by the confinement. The presence of water molecule reorientational dynamics in the tens of ns range that are sufficient to affect the spin-lattice relaxation driven by (1)H dipole-dipole fluctuations should make the local dielectric properties in the protein frequency dependent in a regime relevant to catalytically important kinetic barriers to conformational rearrangements.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Water molecule contributions to proton spin-lattice relaxation in rotationally immobilized proteins.J Magn Reson. 2009 Jul;199(1):68-74. doi: 10.1016/j.jmr.2009.04.001. Epub 2009 Apr 8. J Magn Reson. 2009. PMID: 19394883 Free PMC article.
-
Extreme-values statistics and dynamics of water at protein interfaces.J Phys Chem B. 2011 Nov 10;115(44):12845-58. doi: 10.1021/jp2053426. Epub 2011 Oct 18. J Phys Chem B. 2011. PMID: 21932852
-
Paramagnetic relaxation of protons in rotationally immobilized proteins.J Chem Phys. 2006 Apr 7;124(13):134910. doi: 10.1063/1.2183311. J Chem Phys. 2006. PMID: 16613480
-
Self-diffusion studies by intra- and inter-molecular spin-lattice relaxometry using field-cycling: Liquids, plastic crystals, porous media, and polymer segments.Prog Nucl Magn Reson Spectrosc. 2017 Aug;101:18-50. doi: 10.1016/j.pnmrs.2017.04.001. Epub 2017 Apr 9. Prog Nucl Magn Reson Spectrosc. 2017. PMID: 28844220 Review.
-
Overhauser Dynamic Nuclear Polarization for the Study of Hydration Dynamics, Explained.Methods Enzymol. 2019;615:131-175. doi: 10.1016/bs.mie.2018.09.024. Epub 2018 Dec 11. Methods Enzymol. 2019. PMID: 30638529 Review.
Cited by
-
Markers of low field NMR relaxation features of tissues.Sci Rep. 2024 Oct 22;14(1):24901. doi: 10.1038/s41598-024-74055-7. Sci Rep. 2024. PMID: 39438494 Free PMC article.
-
Practical considerations over spectral quality in solid state NMR spectroscopy of soluble proteins.J Biomol NMR. 2013 Oct;57(2):155-66. doi: 10.1007/s10858-013-9776-0. Epub 2013 Aug 30. J Biomol NMR. 2013. PMID: 23990200
-
Water Dynamics in Highly Concentrated Protein Systems-Insight from Nuclear Magnetic Resonance Relaxometry.Int J Mol Sci. 2023 Feb 17;24(4):4093. doi: 10.3390/ijms24044093. Int J Mol Sci. 2023. PMID: 36835511 Free PMC article.
-
Ion influence on surface water dynamics and proton exchange at protein surfaces - A unified model for transverse and longitudinal NMR relaxation dispersion.J Mol Liq. 2022 Dec 1;367(Pt A):120451. doi: 10.1016/j.molliq.2022.120451. Epub 2022 Sep 24. J Mol Liq. 2022. PMID: 37790165 Free PMC article.
-
Anomalously Rapid Hydration Water Diffusion Dynamics Near DNA Surfaces.J Am Chem Soc. 2015 Sep 23;137(37):12013-23. doi: 10.1021/jacs.5b05813. Epub 2015 Sep 10. J Am Chem Soc. 2015. PMID: 26256693 Free PMC article.
References
-
- Bryant R.G., Mendelson D.A., Lester C.C. The magnetic field dependence of proton spin relaxation in tissues. Magn. Reson. Med. 1991;21:117–126. - PubMed
-
- Koenig S.H., Brown R.D. The importance of the motion of water for magnetic resonance imaging. Invest. Radiol. 1985;20:297–305. - PubMed
-
- Korb J.-P., Bryant R.G. The physical basis for the magnetic field dependence of proton spin-lattice relaxation rates in proteins. J. Chem. Phys. 2001;115:10964–10974.
-
- Nusser W., Kimmich R. Protein backbone fluctuations and NMR field-cycling relaxation spectroscopy. J. Phys. Chem. 1990;94:5637–5639.
-
- Korb J.-P., Bryant R.G. Magnetic field dependence of proton spin-lattice relaxation of confined proteins. C.R. Phys. 2004;5:349–357.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

