The formation of Anthocyanic Vacuolar Inclusions in Arabidopsis thaliana and implications for the sequestration of anthocyanin pigments
- PMID: 20085894
- PMCID: PMC2807924
- DOI: 10.1093/mp/ssp071
The formation of Anthocyanic Vacuolar Inclusions in Arabidopsis thaliana and implications for the sequestration of anthocyanin pigments
Abstract
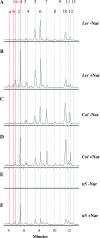
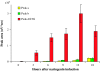
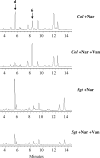
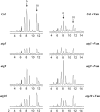
Anthocyanins are flavonoid pigments that accumulate in the large central vacuole of most plants. Inside the vacuole, anthocyanins can be found uniformly distributed or as part of sub-vacuolar pigment bodies, the Anthocyanic Vacuolar Inclusions (AVIs). Using Arabidopsis seedlings grown under anthocyanin-inductive conditions as a model to understand how AVIs are formed, we show here that the accumulation of AVIs strongly correlates with the formation of cyanidin 3-glucoside (C3G) and derivatives. Arabidopsis mutants that fail to glycosylate anthocyanidins at the 5-O position (5gt mutant) accumulate AVIs in almost every epidermal cell of the cotyledons, as compared to wild-type seedlings, where only a small fraction of the cells show AVIs. A similar phenomenon is observed when seedlings are treated with vanadate. Highlighting a role for autophagy in the formation of the AVIs, we show that various mutants that interfere with the autophagic process (atg mutants) display lower numbers of AVIs, in addition to a reduced accumulation of anthocyanins. Interestingly, vanadate increases the numbers of AVIs in the atg mutants, suggesting that several pathways might participate in AVI formation. Taken together, our results suggest novel mechanisms for the formation of sub-vacuolar compartments capable of accumulating anthocyanin pigments.
Keywords: Anthocyanin; autophagy; cyanidin 3-glucoside; vacuolar inclusion; vanadate.
Figures




Similar articles
-
Anthocyanin Vacuolar Inclusions Form by a Microautophagy Mechanism.Plant Cell. 2015 Sep;27(9):2545-59. doi: 10.1105/tpc.15.00589. Epub 2015 Sep 4. Plant Cell. 2015. PMID: 26342015 Free PMC article.
-
Aromatic Decoration Determines the Formation of Anthocyanic Vacuolar Inclusions.Curr Biol. 2017 Apr 3;27(7):945-957. doi: 10.1016/j.cub.2017.02.027. Epub 2017 Mar 16. Curr Biol. 2017. PMID: 28318977 Free PMC article.
-
New insight into the structures and formation of anthocyanic vacuolar inclusions in flower petals.BMC Plant Biol. 2006 Dec 17;6:29. doi: 10.1186/1471-2229-6-29. BMC Plant Biol. 2006. PMID: 17173704 Free PMC article.
-
The role of acyl-glucose in anthocyanin modifications.Molecules. 2014 Nov 14;19(11):18747-66. doi: 10.3390/molecules191118747. Molecules. 2014. PMID: 25405291 Free PMC article. Review.
-
Anthocyanic Vacuolar Inclusions: From Biosynthesis to Storage and Possible Applications.Front Chem. 2022 Jun 28;10:913324. doi: 10.3389/fchem.2022.913324. eCollection 2022. Front Chem. 2022. PMID: 35836677 Free PMC article. Review.
Cited by
-
Arabidopsis ECHIDNA protein is involved in seed coloration, protein trafficking to vacuoles, and vacuolar biogenesis.J Exp Bot. 2020 Jul 6;71(14):3999-4009. doi: 10.1093/jxb/eraa147. J Exp Bot. 2020. PMID: 32201898 Free PMC article.
-
Flavonoids: a review on biosynthesis and transportation mechanism in plants.Funct Integr Genomics. 2023 Jun 27;23(3):212. doi: 10.1007/s10142-023-01147-4. Funct Integr Genomics. 2023. PMID: 37368046 Review.
-
Diverse Physiological Roles of Flavonoids in Plant Environmental Stress Responses and Tolerance.Plants (Basel). 2022 Nov 18;11(22):3158. doi: 10.3390/plants11223158. Plants (Basel). 2022. PMID: 36432887 Free PMC article. Review.
-
Arabidopsis MATE45 antagonizes local abscisic acid signaling to mediate development and abiotic stress responses.Plant Direct. 2018 Oct 12;2(10):e00087. doi: 10.1002/pld3.87. eCollection 2018 Oct. Plant Direct. 2018. PMID: 31245687 Free PMC article.
-
De novo transcriptome revealed genes involved in anthocyanin biosynthesis, transport, and regulation in a mutant of Acer pseudosieboldianum.BMC Genomics. 2022 Aug 8;23(1):567. doi: 10.1186/s12864-022-08815-y. BMC Genomics. 2022. PMID: 35941547 Free PMC article.
References
-
- Bassham DC. Plant autophagy: more than a starvation response. Curr. Opin. Plant Biol. 2007;10:587–593. - PubMed
-
- Buchanan B, Gruissem W, Jones R. Biochemistry and Molecular Biology of Plants. Rockville: ASPP; 2000.
-
- Conn S, Zhang W, Franco C. Anthocyanic vacuolar inclusions (AVIs) selectively bind acylated anthocyanins in Vita vinifera L. (grapevine) suspension culture. Biotech. Ltrs. 2003;25:835–839. - PubMed
-
- Doelling JH, Walker JM, Friedman EM, Thompson AR, Vierstra RD. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J. Biol. Chem. 2002;277:33105–33114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

