Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment
- PMID: 20085927
- PMCID: PMC2858013
- DOI: 10.1093/brain/awp322
Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment
Abstract
Traumatic injury to the central nervous system results in the disruption of the blood brain/spinal barrier, followed by the invasion of cells and other components of the immune system that can aggravate injury and affect subsequent repair and regeneration. Although studies of chronic neuroinflammation in the injured spinal cord of animals are clinically relevant to most patients living with traumatic injury to the brain or spinal cord, very little is known about chronic neuroinflammation, though several studies have tested the role of neuroinflammation in the acute period after injury. The present study characterizes a novel cell preparation method that assesses, quickly and effectively, the changes in the principal immune cell types by flow cytometry in the injured spinal cord, daily for the first 10 days and periodically up to 180 days after spinal cord injury. These data quantitatively demonstrate a novel time-dependent multiphasic response of cellular inflammation in the spinal cord after spinal cord injury and are verified by quantitative stereology of immunolabelled spinal cord sections at selected time points. The early phase of cellular inflammation is comprised principally of neutrophils (peaking 1 day post-injury), macrophages/microglia (peaking 7 days post-injury) and T cells (peaking 9 days post-injury). The late phase of cellular inflammation was detected after 14 days post-injury, peaked after 60 days post-injury and remained detectable throughout 180 days post-injury for all three cell types. Furthermore, the late phase of cellular inflammation (14-180 days post-injury) did not coincide with either further improvements, or new decrements, in open-field locomotor function after spinal cord injury. However, blockade of chemoattractant C5a-mediated inflammation after 14 days post-injury reduced locomotor recovery and myelination in the injured spinal cord, suggesting that the late inflammatory response serves a reparative function. Together, these data provide new insight into cellular inflammation of spinal cord injury and identify a surprising and extended multiphasic response of cellular inflammation. Understanding the role of this multiphasic response in the pathophysiology of spinal cord injury could be critical for the design and implementation of rational therapeutic treatment strategies, including both cell-based and pharmacological interventions.
Figures

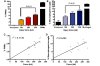



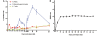

References
-
- Anderson AJ, Robert S, Huang W, Young W, Cotman CW. Activation of complement pathways after contusion-induced spinal cord injury. J Neurotrauma. 2004;21:1831–46. - PubMed
-
- Bagamery K, Kvell K, Landau R, Graham J. Flow cytometric analysis of CD41-labeled platelets isolated by the rapid, one-step OptiPrep method from human blood. Cytometry A. 2005;65:84–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

