Long-term follow-up of autotransplantation trials for multiple myeloma: update of protocols conducted by the intergroupe francophone du myelome, southwest oncology group, and university of arkansas for medical sciences
- PMID: 20085933
- PMCID: PMC2834471
- DOI: 10.1200/JCO.2009.25.6081
Long-term follow-up of autotransplantation trials for multiple myeloma: update of protocols conducted by the intergroupe francophone du myelome, southwest oncology group, and university of arkansas for medical sciences
Erratum in
- J Clin Oncol. 2010 Jul 20;28(21):3543
Abstract
Purpose: The purpose of this study was to update outcomes of autotransplantation trials for myeloma conducted by the Intergroupe Francophone du Myelome (IFM), the Southwest Oncology Group, and the University of Arkansas for Medical Sciences (Total Therapy [TT]).
Methods: IFM90 (N = 194), IFM04 (N = 402), IFM9902 (N = 692), IFM9904 (N = 197), S9321 (N = 817), TT1 (N = 231), TT2 (N = 668), and TT3 (N = 303) were updated, and results were compared with original reports.
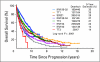
Results: Superior survival with single transplantation versus standard therapy in IFM90 was confirmed (P = .004), and a trend in favor of tandem versus single transplantation was maintained in IFM94 (P = .08). S9321 data were validated, with comparable survival in single transplantation and standard treatment arms (P = .35). A survival benefit from thalidomide maintenance in IFM9902 was not confirmed (P = .39) but emerged for the thalidomide arm of TT2 (P = .04). On multivariate analysis, survival was superior in TT2, TT3, and IFM9902 (all P < .001); tandem transplantations were superior to both single transplantations and standard therapies (P < .001), as were tandem transplantations with added thalidomide versus trials without thalidomide (P < .001). Postrelapse survival (PRS) was superior when initial event-free survival (EFS) exceeded 1280 days and when tandem transplantations had been administered, whereas PRS was shorter when EFS lasted 803 days or less and when trials had included thalidomide and bortezomib.
Conclusion: These long-term follow-up data of transplantation trials provide a crucial framework of reference for outcome reporting of novel agent-based trials reportedly exhibiting remarkable short-term efficacy approaching high-dose therapy results.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Long-term follow-up of tandem autologous stem-cell transplantation in multiple myeloma.J Clin Oncol. 2010 Dec 10;28(35):e741-3; author reply e744-5. doi: 10.1200/JCO.2010.31.5515. Epub 2010 Nov 8. J Clin Oncol. 2010. PMID: 21060027 No abstract available.
References
-
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med. 1996;335:91–97. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. - PubMed
-
- Attal M, Harousseau J-L, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. - PubMed
-
- Barlogie B, Tricot GJ, van Rhee F, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J Haematol. 2006;135:158–164. - PubMed
-
- Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

