NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia
- PMID: 20085940
- PMCID: PMC2834435
- DOI: 10.1200/JCO.2009.24.4590
NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia
Abstract
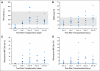
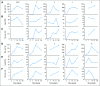
PURPOSE To conduct a pilot study to determine the safety, feasibility, and engraftment of haploidentical natural killer (NK) cell infusions after an immunosuppressive regimen in children with acute myeloid leukemia (AML). PATIENTS AND METHODS Ten patients (0.7 to 21 years old) who had completed chemotherapy and were in first complete remission of AML were enrolled on the Pilot Study of Haploidentical Natural Killer Cell Transplantation for Acute Myeloid Leukemia (NKAML) study. They received cyclophosphamide (60 mg/kg on day -7) and fludarabine (25 mg/m(2)/d on days -6 through -2), followed by killer immunoglobulin-like receptor-human leukocyte antigen (KIR-HLA) mismatched NK cells (median, 29 x 10(6)/kg NK cells) and six doses of interleukin-2 (1 million U/m(2)). NK cell chimerism, phenotyping, and functional assays were performed on days 2, 7, 14, 21, and 28 after transplantation. Results All patients had transient engraftment for a median of 10 days (range, 2 to 189 days) and a significant expansion of KIR-mismatched NK cells (median, 5,800/mL of blood on day 14). Nonhematologic toxicity was limited, with no graft-versus-host disease. Median length of hospitalization was 2 days. With a median follow-up time of 964 days (range, 569 to 1,162 days), all patients remain in remission. The 2-year event-free survival estimate was 100% (95% CI, 63.1% to 100%). CONCLUSION Low-dose immunosuppression followed by donor-recipient inhibitory KIR-HLA mismatched NK cells is well tolerated by patients and results in successful engraftment. We propose to further investigate the efficacy of KIR-mismatched NK cells in a phase II trial as consolidation therapy to decrease relapse without increasing mortality in children with AML.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: Results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. - PubMed
-
- Stevens RF, Hann IM, Wheatley K, et al. Marked improvements in outcome with chemotherapy alone in paediatric acute myeloid leukemia: Results of the United Kingdom Medical Research Council's 10th AML trial. MRC Childhood Leukaemia Working Party. Br J Haematol. 1998;101:130–140. - PubMed
-
- Gibson BE, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

