Toward gene therapy of premature ovarian failure: intraovarian injection of adenovirus expressing human FSH receptor restores folliculogenesis in FSHR(-/-) FORKO mice
- PMID: 20086006
- PMCID: PMC2834408
- DOI: 10.1093/molehr/gaq003
Toward gene therapy of premature ovarian failure: intraovarian injection of adenovirus expressing human FSH receptor restores folliculogenesis in FSHR(-/-) FORKO mice
Abstract
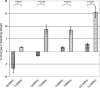
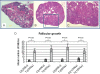
A homozygous missense mutation, C566T, in the follicle stimulation hormone receptor (FSHR) gene has been linked to premature ovarian failure. The disease leads to infertility in a normal karyotype female with an elevated follicle stimulating hormone (FSH) and decreased serum estrogen level. Female mice carrying mutated FSHR gene, called follitropin receptor knockout (FORKO), display similar phenotype and are sterile because of a folliculogenesis block at a primary stage. We investigated the effects of bilateral intra-ovarian injection of an adenovirus expressing a normal copy of human FSHR on the reproductive system of 6-10 weeks female FORKO mice. Ad-LacZ was injected directly into each ovary of the control group. Animals were sacrificed at 2, 4, 8 and 12 weeks post-injection and tissues collected for evaluation. Treated mice showed estrogenic changes in daily vaginal smear whereas control animals remained fixated in the diestrus stage. Histological evaluation showed on average 26 +/- 4 follicles/ovary in treated group with 8 +/- 2 follicles at the antral stage compared with only 5 +/- 2 with zero follicles at antral stage in Ad-LacZ control mice. There was no significant change in serum level of progesterone, however, estrogen level increased 2-3-fold (P < 0.02) and FSH decreased by up to 50% (P < 0.04) in treated animals. FSHR mRNA was detected in the ovaries of the treated group. In conclusion, intra-ovarian injection of an adenovirus expressing human FSHR gene is able to restore FSH responsiveness and reinitiate ovarian folliculogenesis as well as resume estrogen production in female FORKO mice. Ad-LacZ injections indicate the absence of systemic viral dissemination or germ line transmission of adenovirus DNA to offspring.
Figures




References
-
- Aittomaki K, Lucerna JL, Pakarinen P, Sistonen P, Tapanainen JS, Gromoll J, Kaskikari R, Sankila EM, Lehvaslaiho H, Engel AR. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. - PubMed
-
- Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191:1621–1631. - PubMed
-
- Al-Hendy A, Wang H, Salama SA. Towards Gene Therapy of Ovarian Failure: Intraovarian injected adenovirus successfully transduced granulosa and stromal but not germ cells. J Soc Gynecol Investig. 2005;12 Ref Type: Abstract.
-
- Allen LA, Achermann JC, Pakarinen P, Kotlar TJ, Huhtaniemi IT, Jameson JL, Cheetham TD, Ball SG. A novel loss of function mutation in exon 10 of the FSH receptor gene causing hypergonadotrophic hypogonadism: clinical and molecular characteristics. Hum Reprod. 2003;18:251–256. - PubMed
-
- Balla A, Danilovich N, Yang Y, Sairam MR. Dynamics of ovarian development in the FORKO immature mouse: structural and functional implications for ovarian reserve. Biol Reprod. 2003;69:1281–1293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

