Proteolysis of mutant huntingtin produces an exon 1 fragment that accumulates as an aggregated protein in neuronal nuclei in Huntington disease
- PMID: 20086007
- PMCID: PMC2838303
- DOI: 10.1074/jbc.M109.075028
Proteolysis of mutant huntingtin produces an exon 1 fragment that accumulates as an aggregated protein in neuronal nuclei in Huntington disease
Abstract
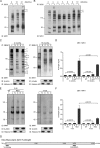
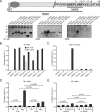
Huntingtin proteolysis has been implicated in the molecular pathogenesis of Huntington disease (HD). Despite an intense effort, the identity of the pathogenic smallest N-terminal fragment has not been determined. Using a panel of anti-huntingtin antibodies, we employed an unbiased approach to generate proteolytic cleavage maps of mutant and wild-type huntingtin in the HdhQ150 knock-in mouse model of HD. We identified 14 prominent N-terminal fragments, which, in addition to the full-length protein, can be readily detected in cytoplasmic but not nuclear fractions. These fragments were detected at all ages and are not a consequence of the pathogenic process. We demonstrated that the smallest fragment is an exon 1 huntingtin protein, known to contain a potent nuclear export signal. Prior to the onset of behavioral phenotypes, the exon 1 protein, and possibly other small fragments, accumulate in neuronal nuclei in the form of a detergent insoluble complex, visualized as diffuse granular nuclear staining in tissue sections. This methodology can be used to validate the inhibition of specific proteases as therapeutic targets for HD by pharmacological or genetic approaches.
Figures


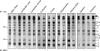



References
-
- Bates G. P., Harper P. S., Jones A. L. (eds) (2002) Huntington's Disease, Oxford University Press, Oxford
-
- Harjes P., Wanker E. E. (2003) Trends Biochem. Sci. 28, 425–433 - PubMed
-
- Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S. W., Bates G. P. (1996) Cell 87, 493–506 - PubMed
-
- Li H., Li S. H., Cheng A. L., Mangiarini L., Bates G. P., Li X. J. (1999) Hum. Mol. Genet. 8, 1227–1236 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

