Live cell fluorescence resonance energy transfer predicts an altered molecular association of heterologous PrPSc with PrPC
- PMID: 20086009
- PMCID: PMC2838318
- DOI: 10.1074/jbc.M109.058107
Live cell fluorescence resonance energy transfer predicts an altered molecular association of heterologous PrPSc with PrPC
Abstract
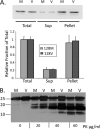
Prion diseases result from the accumulation of a misfolded isoform (PrP(Sc)) of the normal host prion protein (PrP(C)). PrP(Sc) propagates by templating its conformation onto resident PrP(C) to generate new PrP(Sc). Although the nature of the PrP(Sc)-PrP(C) complex is unresolved, certain segments or specific residues are thought to feature critically in its formation. The polymorphic residue 129 is one such site under considerable study. We combined transmission studies with a novel live cell yeast-based fluorescence resonance energy transfer (FRET) system that models the molecular association of PrP in a PrP(Sc)-like state, as a way to explore the role of residue 129 in this process. We show that a reduction in efficiency of prion transmission between donor PrP(Sc) and recipient PrP(C) that are mismatched at residue 129 correlates with a reduction in FRET between PrP-129M and PrP-129V in our yeast model. We further show that this effect depends on the different secondary structure propensities of Met and Val, rather than the specific amino acids. Finally, introduction of the disease-associated P101L mutation (mouse- equivalent) abolished FRET with wild-type mouse PrP, whereas mutant PrP-P101L displayed high FRET with homologous PrP-P101L, as long as residue 129 matched. These studies provide the first evidence for a physical alteration in the molecular association of PrP molecules differing in one or more residues, and they further predict that the different secondary structure propensities of Met and Val define the impaired association observed between PrP(Sc) and PrP(C) mismatched at residue 129.
Figures


Similar articles
-
The AGAAAAGA palindrome in PrP is required to generate a productive PrPSc-PrPC complex that leads to prion propagation.J Biol Chem. 2005 Jul 22;280(29):27236-43. doi: 10.1074/jbc.M413441200. Epub 2005 May 25. J Biol Chem. 2005. PMID: 15917252
-
Identifying key components of the PrPC-PrPSc replicative interface.J Biol Chem. 2008 Dec 5;283(49):34021-8. doi: 10.1074/jbc.M804475200. Epub 2008 Sep 30. J Biol Chem. 2008. PMID: 18826953 Free PMC article.
-
Molecular biology and pathology of prion strains in sporadic human prion diseases.Acta Neuropathol. 2011 Jan;121(1):79-90. doi: 10.1007/s00401-010-0761-3. Epub 2010 Nov 7. Acta Neuropathol. 2011. PMID: 21058033 Free PMC article. Review.
-
Chemical induction of misfolded prion protein conformers in cell culture.J Biol Chem. 2010 Apr 2;285(14):10415-23. doi: 10.1074/jbc.M109.045112. Epub 2009 Dec 2. J Biol Chem. 2010. PMID: 19955177 Free PMC article.
-
Molecular Mechanism of the Misfolding and Oligomerization of the Prion Protein: Current Understanding and Its Implications.Biochemistry. 2015 Jul 28;54(29):4431-42. doi: 10.1021/acs.biochem.5b00605. Epub 2015 Jul 17. Biochemistry. 2015. PMID: 26171558 Review.
Cited by
-
Caprine PrP variants harboring Asp-146, His-154 and Gln-211 alleles display reduced convertibility upon interaction with pathogenic murine prion protein in scrapie infected cells.Prion. 2016 Sep 2;10(5):391-408. doi: 10.1080/19336896.2016.1199312. Prion. 2016. PMID: 27537339 Free PMC article.
-
FRET Microscopy in Yeast.Biosensors (Basel). 2019 Oct 11;9(4):122. doi: 10.3390/bios9040122. Biosensors (Basel). 2019. PMID: 31614546 Free PMC article. Review.
-
Generation of human chronic wasting disease in transgenic mice.Acta Neuropathol Commun. 2021 Sep 26;9(1):158. doi: 10.1186/s40478-021-01262-y. Acta Neuropathol Commun. 2021. PMID: 34565488 Free PMC article.
-
Molecular pathology of human prion disease.Acta Neuropathol. 2011 Jan;121(1):69-77. doi: 10.1007/s00401-010-0735-5. Epub 2010 Aug 8. Acta Neuropathol. 2011. PMID: 20694796 Free PMC article. Review.
-
Impaired transmissibility of atypical prions from genetic CJDG114V.Neurol Genet. 2018 Aug 7;4(4):e253. doi: 10.1212/NXG.0000000000000253. eCollection 2018 Aug. Neurol Genet. 2018. PMID: 30109268 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

