Loss of mitochondrial DNA in the yeast cardiolipin synthase crd1 mutant leads to up-regulation of the protein kinase Swe1p that regulates the G2/M transition
- PMID: 20086012
- PMCID: PMC2856246
- DOI: 10.1074/jbc.M110.100784
Loss of mitochondrial DNA in the yeast cardiolipin synthase crd1 mutant leads to up-regulation of the protein kinase Swe1p that regulates the G2/M transition
Abstract
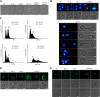
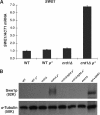
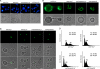
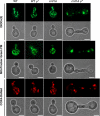
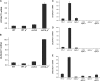
The anionic phospholipid cardiolipin and its precursor phosphatidylglycerol are synthesized and localized in the mitochondrial inner membrane of eukaryotes. They are required for structural integrity and optimal activities of a large number of mitochondrial proteins and complexes. Previous studies showed that loss of anionic phospholipids leads to cell inviability in the absence of mitochondrial DNA. However, the mechanism linking loss of anionic phospholipids to petite lethality was unclear. To elucidate the mechanism, we constructed a crd1Deltarho degrees mutant, which is viable and mimics phenotypes of pgs1Delta in the petite background. We found that loss of cardiolipin in rho degrees cells leads to elevated expression of Swe1p, a morphogenesis checkpoint protein. Moreover, the retrograde pathway is activated in crd1Deltarho degrees cells, most likely due to the exacerbation of mitochondrial dysfunction. Interestingly, the expression of SWE1 is dependent on retrograde regulation as elevated expression of SWE1 is suppressed by deletion of RTG2 or RTG3. Taken together, these findings indicate that activation of the retrograde pathway leads to up-regulation of SWE1 in crd1Deltarho degrees cells. These results suggest that anionic phospholipids are required for processes that are essential for normal cell division in rho degrees cells.
Figures
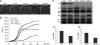
Similar articles
-
Absence of cardiolipin results in temperature sensitivity, respiratory defects, and mitochondrial DNA instability independent of pet56.J Biol Chem. 2004 Jul 30;279(31):32294-300. doi: 10.1074/jbc.M403275200. Epub 2004 May 29. J Biol Chem. 2004. PMID: 15169766
-
Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function.J Biol Chem. 2000 Jul 21;275(29):22387-94. doi: 10.1074/jbc.M909868199. J Biol Chem. 2000. PMID: 10777514
-
Regulation of cardiolipin synthase levels in Saccharomyces cerevisiae.Yeast. 2006 Mar;23(4):279-91. doi: 10.1002/yea.1352. Yeast. 2006. PMID: 16544270 Free PMC article.
-
Cardiolipin synthase from yeast.Biochim Biophys Acta. 1997 Sep 4;1348(1-2):201-6. doi: 10.1016/s0005-2760(97)00117-3. Biochim Biophys Acta. 1997. PMID: 9370334 Review.
-
Cardiolipin at the heart of stress response across kingdoms.Plant Signal Behav. 2014;9(9):e29228. doi: 10.4161/psb.29228. Plant Signal Behav. 2014. PMID: 25763690 Free PMC article. Review.
Cited by
-
Num1 anchors mitochondria to the plasma membrane via two domains with different lipid binding specificities.J Cell Biol. 2016 Jun 6;213(5):513-24. doi: 10.1083/jcb.201511021. Epub 2016 May 30. J Cell Biol. 2016. PMID: 27241910 Free PMC article.
-
Gene dosage adaptations to mtDNA depletion and mitochondrial protein stress in budding yeast.G3 (Bethesda). 2024 Feb 7;14(2):jkad272. doi: 10.1093/g3journal/jkad272. G3 (Bethesda). 2024. PMID: 38126114 Free PMC article.
-
Deletion of the cardiolipin-specific phospholipase Cld1 rescues growth and life span defects in the tafazzin mutant: implications for Barth syndrome.J Biol Chem. 2014 Feb 7;289(6):3114-25. doi: 10.1074/jbc.M113.529487. Epub 2013 Dec 8. J Biol Chem. 2014. PMID: 24318983 Free PMC article.
-
Cardiolipin deficiency causes triacylglycerol accumulation in Saccharomyces cerevisiae.Mol Cell Biochem. 2017 Oct;434(1-2):89-103. doi: 10.1007/s11010-017-3039-4. Epub 2017 Apr 21. Mol Cell Biochem. 2017. PMID: 28432553
-
A conserved mechanism for mitochondria-dependent dynein anchoring.Mol Biol Cell. 2019 Mar 1;30(5):691-702. doi: 10.1091/mbc.E18-07-0466. Epub 2019 Jan 16. Mol Biol Cell. 2019. PMID: 30649994 Free PMC article.
References
-
- Schlame M., Rua D., Greenberg M. L. (2000) Prog. Lipid Res. 39, 257–288 - PubMed
-
- Hoch F. L. (1992) Biochim. Biophys. Acta 1113, 71–133 - PubMed
-
- Yu C. A., Yu L. (1980) Biochemistry 19, 5715–5720 - PubMed
-
- Drees M., Beyer K. (1988) Biochemistry 27, 8584–8591 - PubMed
-
- Beyer K., Nuscher B. (1996) Biochemistry 35, 15784–15790 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

