Hepatocyte nuclear factor 4{alpha} regulates rifampicin-mediated induction of CYP2C genes in primary cultures of human hepatocytes
- PMID: 20086032
- PMCID: PMC2845933
- DOI: 10.1124/dmd.109.030387
Hepatocyte nuclear factor 4{alpha} regulates rifampicin-mediated induction of CYP2C genes in primary cultures of human hepatocytes
Abstract
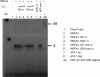
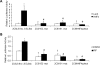
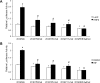
CYP2C enzymes are expressed constitutively and comprise approximately 20% of the total cytochrome P450 in human liver. However, the factors influencing the transcriptional regulation of the CYP2C subfamily have only been addressed recently. In the present study, we used primary cultures of human hepatocytes to investigate the role of HNF4alpha in the pregnane X receptor (PXR)/rifampicin-mediated up-regulation of CYP2C8, CYP2C9, and CYP2C19 gene expression. We first identified new proximal cis-acting HNF4alpha sites in the proximal CYP2C8 promoter [at -181 base pairs (bp) from the translation start site] and the CYP2C9 promoter (at -211 bp). Both sites bound HNF4alpha in gel shift assays. Thus, these and recent studies identified a total of three HNF4alpha sites in the CYP2C9 promoter and two in the CYP2C8 promoter. Mutational studies showed that the HNF4alpha sites are needed for up-regulation of the CYP2C8 and CYP2C9 promoters by rifampicin. Furthermore, silencing of HNF4alpha abolished transactivation of the CYP2C8 and CYP2C9 promoters by rifampicin. Constitutive promoter activity was also decreased. Quantitative polymerase chain reaction analysis demonstrated that silencing HNF4alpha reduced the constitutive expression of CYP2C8 (53%), CYP2C9 (55%), and CYP2C19 (43%) mRNAs and significantly decreased the magnitude of the rifampicin-mediated induction of CYP2C8 (6.6- versus 2.7-fold), CYP2C9 (3- versus 1.5-fold), and CYP2C19 (1.8- versus 1.1-fold). These results provide clear evidence that HNF4alpha contributes to the constitutive expression of the human CYP2C genes and is also important for up-regulation by the PXR agonist rifampicin.
Figures
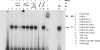
Similar articles
-
Rifampicin induction of CYP3A4 requires pregnane X receptor cross talk with hepatocyte nuclear factor 4alpha and coactivators, and suppression of small heterodimer partner gene expression.Drug Metab Dispos. 2006 May;34(5):756-64. doi: 10.1124/dmd.105.007575. Epub 2006 Feb 2. Drug Metab Dispos. 2006. PMID: 16455805 Free PMC article.
-
Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha.Mol Pharmacol. 2005 Sep;68(3):747-57. doi: 10.1124/mol.105.013169. Epub 2005 Jun 2. Mol Pharmacol. 2005. PMID: 15933212
-
The nuclear receptors constitutive androstane receptor and pregnane X receptor cross-talk with hepatic nuclear factor 4alpha to synergistically activate the human CYP2C9 promoter.J Pharmacol Exp Ther. 2005 Sep;314(3):1125-33. doi: 10.1124/jpet.105.087072. Epub 2005 May 26. J Pharmacol Exp Ther. 2005. PMID: 15919766
-
Valproic acid induces CYP3A4 and MDR1 gene expression by activation of constitutive androstane receptor and pregnane X receptor pathways.Drug Metab Dispos. 2007 Jul;35(7):1032-41. doi: 10.1124/dmd.106.014456. Epub 2007 Mar 28. Drug Metab Dispos. 2007. PMID: 17392393
-
The transcriptional regulation of the human CYP2C genes.Curr Drug Metab. 2009 Jul;10(6):567-78. doi: 10.2174/138920009789375397. Epub 2009 Jul 15. Curr Drug Metab. 2009. PMID: 19702536 Free PMC article. Review.
Cited by
-
PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19.Pharmacogenet Genomics. 2012 Feb;22(2):159-65. doi: 10.1097/FPC.0b013e32834d4962. Pharmacogenet Genomics. 2012. PMID: 22027650 Free PMC article.
-
Peroxisome proliferator-activated receptor alpha, PPARα, directly regulates transcription of cytochrome P450 CYP2C8.Front Pharmacol. 2015 Nov 4;6:261. doi: 10.3389/fphar.2015.00261. eCollection 2015. Front Pharmacol. 2015. PMID: 26582990 Free PMC article.
-
Human CYP2C8 is post-transcriptionally regulated by microRNAs 103 and 107 in human liver.Mol Pharmacol. 2012 Sep;82(3):529-40. doi: 10.1124/mol.112.078386. Epub 2012 Jun 20. Mol Pharmacol. 2012. PMID: 22723340 Free PMC article.
-
Med25 is required for RNA polymerase II recruitment to specific promoters, thus regulating xenobiotic and lipid metabolism in human liver.Mol Cell Biol. 2011 Feb;31(3):466-81. doi: 10.1128/MCB.00847-10. Epub 2010 Dec 6. Mol Cell Biol. 2011. PMID: 21135126 Free PMC article.
-
Genome-wide discovery of drug-dependent human liver regulatory elements.PLoS Genet. 2014 Oct 2;10(10):e1004648. doi: 10.1371/journal.pgen.1004648. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25275310 Free PMC article.
References
-
- Akiyama TE, Gonzalez FJ. (2003) Regulation of P450 genes by liver-enriched transcription factors and nuclear receptors. Biochim Biophys Acta 1619:223–234 - PubMed
-
- Bort R, Gómez-Lechón MJ, Castell JV, Jover R. (2004) Role of hepatocyte nuclear factor 3γ in the expression of human CYP2C genes. Arch Biochem Biophys 426:63–72 - PubMed
-
- Chen Y, Ferguson SS, Negishi M, Goldstein JA. (2003) Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol 64:316–324 - PubMed
-
- Chen Y, Ferguson SS, Negishi M, Goldstein JA. (2004) Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane X receptor. J Pharmacol Exp Ther 308:495–501 - PubMed
-
- Chen Y, Kissling G, Negishi M, Goldstein JA. (2005) The nuclear receptors constitutive androstane receptor and pregnane X receptor cross-talk with hepatic nuclear factor 4α to synergistically activate the human CYP2C9 promoter. J Pharmacol Exp Ther 314:1125–1133 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

