Lens gap junctions in growth, differentiation, and homeostasis
- PMID: 20086076
- PMCID: PMC4627646
- DOI: 10.1152/physrev.00034.2009
Lens gap junctions in growth, differentiation, and homeostasis
Abstract
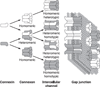
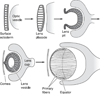
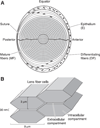
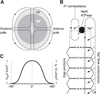
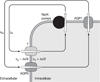
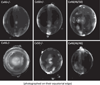
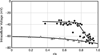
The cells of most mammalian organs are connected by groups of cell-to-cell channels called gap junctions. Gap junction channels are made from the connexin (Cx) family of proteins. There are at least 20 isoforms of connexins, and most tissues express more than 1 isoform. The lens is no exception, as it expresses three isoforms: Cx43, Cx46, and Cx50. A common role for all gap junctions, regardless of their Cx composition, is to provide a conduit for ion flow between cells, thus creating a syncytial tissue with regard to intracellular voltage and ion concentrations. Given this rather simple role of gap junctions, a persistent question has been: Why are there so many Cx isoforms and why do tissues express more than one isoform? Recent studies of lens Cx knockout (KO) and knock in (KI) lenses have begun to answer these questions. To understand these roles, one must first understand the physiological requirements of the lens. We therefore first review the development and structure of the lens, its numerous transport systems, how these systems are integrated to generate the lens circulation, the roles of the circulation in lens homeostasis, and finally the roles of lens connexins in growth, development, and the lens circulation.
Figures








References
-
- Addison PK, Berry V, Holden KR, Espinal D, Rivera B, Su H, Srivastava AK, Bhattacharya SS. A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis. 2006;12:791–795. - PubMed
-
- Al-Ghoul KJ, Kirk T, Kuszak AJ, Zoltoski RK, Shiels A, Kuszak JR. Lens structure in MIP-deficient mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;273:714–730. - PubMed
-
- Alvarez LJ, Candia OA, Polikoff LA. Beta-adrenergic stimulation of Na(+)-K(+)-2Cl(−) cotransport activity in the rabbit lens. Exp Eye Res. 2003;76:61–70. - PubMed
-
- Alvarez LJ, Candia OA, Turner HC, Polikoff LA. Localization of a Na(+)-K(+)-2Cl(−) cotransporter in the rabbit lens. Exp Eye Res. 2001;73:669–680. - PubMed
-
- Alvarez LJ, Candia OA, Wolosin JM. Evidence for parallel Na(+)-H(+) and Na(+)-dependent Cl(−)-HCO3(−) exchangers in cultured bovine lens cells. Exp Eye Res. 1992;55:747–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

