Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection
- PMID: 20086083
- PMCID: PMC2849400
- DOI: 10.1128/IAI.00743-09
Burkholderia cenocepacia creates an intramacrophage replication niche in zebrafish embryos, followed by bacterial dissemination and establishment of systemic infection
Abstract
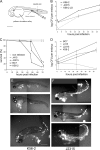
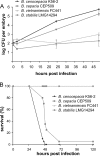
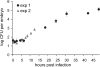
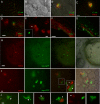
Bacteria belonging to the "Burkholderia cepacia complex" (Bcc) often cause fatal pulmonary infections in cystic fibrosis patients, yet little is know about the underlying molecular mechanisms. These Gram-negative bacteria can adopt an intracellular lifestyle, although their ability to replicate intracellularly has been difficult to demonstrate. Here we show that Bcc bacteria survive and multiply in macrophages of zebrafish embryos. Local dissemination by nonlytic release from infected cells was followed by bacteremia and extracellular replication. Burkholderia cenocepacia isolates belonging to the epidemic electrophoretic type 12 (ET12) lineage were highly virulent for the embryos; intravenous injection of <10 bacteria of strain K56-2 killed embryos within 3 days. However, small but significant differences between the clonal ET12 isolates K56-2, J2315, and BC7 were evident. In addition, the innate immune response in young embryos was sufficiently developed to control infection with other less virulent Bcc strains, such as Burkholderia vietnamiensis FC441 and Burkholderia stabilis LMG14294. A K56-2 cepR quorum-sensing regulator mutant was highly attenuated, and its ability to replicate and spread to neighboring cells was greatly reduced. Our data indicate that the zebrafish embryo is an excellent vertebrate model to dissect the molecular basis of intracellular replication and the early innate immune responses in this intricate host-pathogen interaction.
Figures

Similar articles
-
Burkholderia multivorans survival and trafficking within macrophages.J Med Microbiol. 2013 Feb;62(Pt 2):173-184. doi: 10.1099/jmm.0.051243-0. Epub 2012 Oct 25. J Med Microbiol. 2013. PMID: 23105020
-
The afc antifungal activity cluster, which is under tight regulatory control of ShvR, is essential for transition from intracellular persistence of Burkholderia cenocepacia to acute pro-inflammatory infection.PLoS Pathog. 2018 Dec 4;14(12):e1007473. doi: 10.1371/journal.ppat.1007473. eCollection 2018 Dec. PLoS Pathog. 2018. PMID: 30513124 Free PMC article.
-
Mutation of hmgA, encoding homogentisate 1,2-dioxygenase, is responsible for pyomelanin production but does not impact the virulence of Burkholderia cenocepacia in a chronic granulomatous disease mouse lung infection.Microbiol Spectr. 2024 Jul 2;12(7):e0041024. doi: 10.1128/spectrum.00410-24. Epub 2024 May 29. Microbiol Spectr. 2024. PMID: 38809005 Free PMC article.
-
Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence.Clin Microbiol Infect. 2010 Jul;16(7):821-30. doi: 10.1111/j.1469-0691.2010.03237.x. Clin Microbiol Infect. 2010. PMID: 20880411 Review.
-
A decade of Burkholderia cenocepacia virulence determinant research.Infect Immun. 2010 Oct;78(10):4088-100. doi: 10.1128/IAI.00212-10. Epub 2010 Jul 19. Infect Immun. 2010. PMID: 20643851 Free PMC article. Review.
Cited by
-
Identification of the flagellin glycosylation system in Burkholderia cenocepacia and the contribution of glycosylated flagellin to evasion of human innate immune responses.J Biol Chem. 2014 Jul 4;289(27):19231-44. doi: 10.1074/jbc.M114.562603. Epub 2014 May 19. J Biol Chem. 2014. PMID: 24841205 Free PMC article.
-
Use of Synthetic Hybrid Strains To Determine the Role of Replicon 3 in Virulence of the Burkholderia cepacia Complex.Appl Environ Microbiol. 2017 Jun 16;83(13):e00461-17. doi: 10.1128/AEM.00461-17. Print 2017 Jul 1. Appl Environ Microbiol. 2017. PMID: 28432094 Free PMC article.
-
Autophagy stimulation by rapamycin suppresses lung inflammation and infection by Burkholderia cenocepacia in a model of cystic fibrosis.Autophagy. 2011 Nov;7(11):1359-70. doi: 10.4161/auto.7.11.17660. Epub 2011 Nov 1. Autophagy. 2011. PMID: 21997369 Free PMC article.
-
Macrophage-Microbe Interactions: Lessons from the Zebrafish Model.Front Immunol. 2017 Dec 1;8:1703. doi: 10.3389/fimmu.2017.01703. eCollection 2017. Front Immunol. 2017. PMID: 29250076 Free PMC article. Review.
-
Selective autophagy: the rise of the zebrafish model.Autophagy. 2021 Nov;17(11):3297-3305. doi: 10.1080/15548627.2020.1853382. Epub 2020 Dec 15. Autophagy. 2021. PMID: 33228439 Free PMC article. Review.
References
-
- Boshra, H., J. Li, and J. O. Sunyer. 2006. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 20:239-262. - PubMed
-
- Brannon, M. K., J. M. Davis, J. R. Mathias, C. J. Hall, J. C. Emerson, P. S. Crosier, A. Huttenlocher, L. Ramakrishnan, and S. M. Moskowitz. 2009. Pseudomonas aeruginosa type III secretion system interacts with phagocytes to modulate systemic infection of zebrafish embryos. Cell. Microbiol. 11:755-768. - PMC - PubMed
-
- Bylund, J., L. A. Burgess, P. Cescutti, R. K. Ernst, and D. P. Speert. 2006. Exopolysaccharides from Burkholderia cenocepacia inhibit neutrophil chemotaxis and scavenge reactive oxygen species. J. Biol. Chem. 281:2526-2532. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

