Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans
- PMID: 20086085
- PMCID: PMC2849408
- DOI: 10.1128/IAI.01021-09
Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans
Abstract
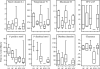
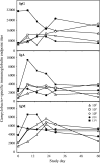
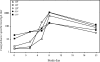
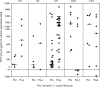
A human Campylobacter jejuni infection model provided controlled exposure to assess vaccine efficacy and investigate protective immunity for this important diarrheal pathogen. A well-characterized outbreak strain, C. jejuni 81-176, was investigated using a volunteer experimental infection model to evaluate the dose range and duration of protection. Healthy Campylobacter-seronegative adults received C. jejuni strain 81-176 via oral inoculation of 10(5), 10(7), or 10(9) CFU (5 adults/dose), which was followed by clinical and immunological monitoring. Based on dose range clinical outcomes, the 10(9)-CFU dose (n = 31) was used to assess homologous protection at 28 to 49 days (short-term veterans [STV]; n = 8) or 1 year (long-term veterans [LTV]; n = 7) after primary infection. An illness dose effect was observed for naïve subjects (with lower doses, 40 to 60% of the subjects were ill; with the 10(9)-CFU dose, 92% of the subjects were ill) along with complete protection for the STV group and attenuated illness for the LTV group (57%). Partial resistance to colonization was seen in STV (25% of the subjects were not infected; 3-log-lower maximum excretion level). Systemic and mucosal immune responses were robust in naïve subjects irrespective of the dose or the severity of illness. In contrast, in STV there was a lack of circulating antibody-secreting cells (ASC), reflecting the local mucosal effector responses. LTV exhibited comparable ASC responses to primary infection, and anamnestic fecal IgA responses likely contributed to self-resolving illness prior to antibiotic treatment. Campylobacter antigen-dependent production of gamma interferon by peripheral blood mononuclear cells was strongly associated with protection from illness, supporting the hypothesis that TH1 polarization has a primary role in acquired immunity to C. jejuni. This study revealed a C. jejuni dose-related increase in campylobacteriosis rates, evidence of complete short-term protection that waned with time, and immune response patterns associated with protection.
Figures

Similar articles
-
Lack of homologous protection against Campylobacter jejuni CG8421 in a human challenge model.Clin Infect Dis. 2013 Oct;57(8):1106-13. doi: 10.1093/cid/cit454. Epub 2013 Jul 9. Clin Infect Dis. 2013. PMID: 23840001 Free PMC article. Clinical Trial.
-
Campylobacter jejuni strain CG8421: a refined model for the study of Campylobacteriosis and evaluation of Campylobacter vaccines in human subjects.Clin Infect Dis. 2009 Nov 15;49(10):1512-9. doi: 10.1086/644622. Clin Infect Dis. 2009. PMID: 19842970 Clinical Trial.
-
Oral immunization with cholera toxin provides protection against Campylobacter jejuni in an adult mouse intestinal colonization model.mBio. 2013 May 7;4(3):e00246-13. doi: 10.1128/mBio.00246-13. mBio. 2013. PMID: 23653448 Free PMC article.
-
A capsule conjugate vaccine approach to prevent diarrheal disease caused by Campylobacter jejuni.Hum Vaccin Immunother. 2014;10(6):1499-504. doi: 10.4161/hv.27985. Epub 2014 Mar 14. Hum Vaccin Immunother. 2014. PMID: 24632556 Free PMC article. Review.
-
Vaccines against Campylobacter jejuni.J Infect Dis. 1997 Dec;176 Suppl 2:S183-8. doi: 10.1086/513791. J Infect Dis. 1997. PMID: 9396708 Review.
Cited by
-
Campylobacter in Africa - A specific viewpoint.Eur J Microbiol Immunol (Bp). 2023 Dec 5;13(4):107-124. doi: 10.1556/1886.2023.00043. Print 2023 Dec 21. Eur J Microbiol Immunol (Bp). 2023. PMID: 38051352 Free PMC article. Review.
-
Visitors to a Tropical Marine Beach Show Evidence of Immunoconversions to Multiple Waterborne Pathogens.Front Public Health. 2019 Aug 19;7:231. doi: 10.3389/fpubh.2019.00231. eCollection 2019. Front Public Health. 2019. PMID: 31482082 Free PMC article.
-
Campylobacter infection promotes IFNγ-dependent intestinal pathology via ILC3 to ILC1 conversion.Mucosal Immunol. 2021 May;14(3):703-716. doi: 10.1038/s41385-020-00353-8. Epub 2020 Nov 19. Mucosal Immunol. 2021. PMID: 33214656 Free PMC article.
-
Lack of homologous protection against Campylobacter jejuni CG8421 in a human challenge model.Clin Infect Dis. 2013 Oct;57(8):1106-13. doi: 10.1093/cid/cit454. Epub 2013 Jul 9. Clin Infect Dis. 2013. PMID: 23840001 Free PMC article. Clinical Trial.
-
Polyphosphate and associated enzymes as global regulators of stress response and virulence in Campylobacter jejuni.World J Gastroenterol. 2016 Sep 7;22(33):7402-14. doi: 10.3748/wjg.v22.i33.7402. World J Gastroenterol. 2016. PMID: 27672264 Free PMC article. Review.
References
-
- Ailes, E., L. Demma, S. Hurd, J. Hatch, T. F. Jones, D. Vugia, A. Cronquist, M. Tobin-D'Angelo, K. Larson, E. Laine, K. Edge, S. Zansky, and E. Scallan. 2008. Continued decline in the incidence of Campylobacter infections, FoodNet 1996-2006. Foodborne Pathog. Dis. 5:329-337. - PubMed
-
- Altekruse, S. F., D. L. Swerdlow, and N. J. Stern. 1998. Microbial food borne pathogens. Campylobacter jejuni. Vet. Clin. N. Am. 14:31-40. - PubMed
-
- Baqar, S., B. Rice, L. Lee, A. L. Bourgeois, A. N. El Din, D. R. Tribble, G. P. Heresi, A. S. Mourad, and J. R. Murphy. 2001. Campylobacter jejuni enteritis. Clin. Infect. Dis. 33:901-905. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

