Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis
- PMID: 20086090
- PMCID: PMC2825929
- DOI: 10.1128/IAI.00925-09
Polysaccharide capsule and sialic acid-mediated regulation promote biofilm-like intracellular bacterial communities during cystitis
Abstract
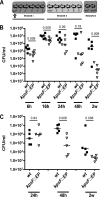
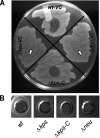
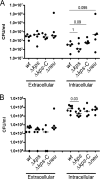
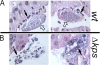
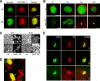
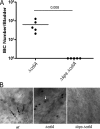
Uropathogenic Escherichia coli (UPEC) is the leading cause of urinary tract infections (UTIs). A murine UTI model has revealed an infection cascade whereby UPEC undergoes cycles of invasion of the bladder epithelium, intracellular proliferation in polysaccharide-containing biofilm-like masses called intracellular bacterial communities (IBC), and then dispersal into the bladder lumen to initiate further rounds of epithelial colonization and invasion. We predicted that the UPEC K1 polysaccharide capsule is a key constituent of the IBC matrix. Compared to prototypic E. coli K1 strain UTI89, a capsule assembly mutant had a fitness defect in functionally TLR4(+) and TLR4(-) mice, suggesting a protective role of capsule in inflamed and noninflamed hosts. K1 capsule assembly and synthesis mutants had dramatically reduced IBC formation, demonstrating the common requirement for K1 polysaccharide in IBC development. The capsule assembly mutant appeared dispersed in the cytoplasm of the bladder epithelial cells and failed to undergo high-density intracellular replication during later stages of infection, when the wild-type strain continued to form serial generations of IBC. Deletion of the sialic acid regulator gene nanR partially restored IBC formation in the capsule assembly mutant. These data suggest that capsule is necessary for efficient IBC formation and that aberrant sialic acid accumulation, resulting from disruption of K1 capsule assembly, produces a NanR-mediated defect in intracellular proliferation and IBC development. Together, these data demonstrate the complex but important roles of UPEC polysaccharide encapsulation and sialic acid signaling in multiple stages of UTI pathogenesis.
Figures
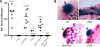
References
-
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
-
- Anderson, J. B., F. Parivar, G. Lee, T. B. Wallington, A. G. MacIver, R. A. Bradbrook, and J. C. Gingell. 1989. The enigma of interstitial cystitis—an autoimmune disease? Br. J. Urol. 63:58-63. - PubMed
-
- Aoki, S. K., R. Pamma, A. D. Hernday, J. E. Bickham, B. A. Braaten, and D. A. Low. 2005. Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245-1248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

