Characterization of PXK as a protein involved in epidermal growth factor receptor trafficking
- PMID: 20086096
- PMCID: PMC2838084
- DOI: 10.1128/MCB.01105-09
Characterization of PXK as a protein involved in epidermal growth factor receptor trafficking
Abstract
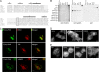
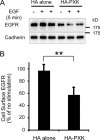
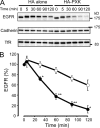
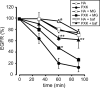
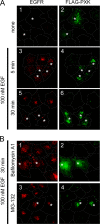
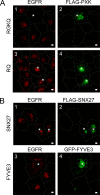
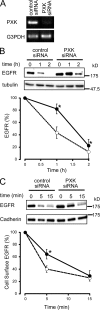
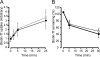
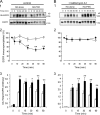
The phox homology (PX) domain is a phosphoinositide-binding module that typically binds phosphatidylinositol 3-phosphate. Out of 47 mammalian proteins containing PX domains, more than 30 are denoted sorting nexins and several of these have been implicated in internalization of cell surface proteins to the endosome, where phosphatidylinositol-3-phosphate is concentrated. Here we investigated a multimodular protein termed PXK, composed of a PX domain, a protein kinase-like domain, and a WASP homology 2 domain. We show that the PX domain of PXK localizes this protein to the endosomal membrane via binding to phosphatidylinositol 3-phosphate. PXK expression in COS7 cells accelerated the ligand-induced internalization and degradation of epidermal growth factor receptors by a mechanism requiring phosphatidylinositol 3-phosphate binding but not involving the WASP homology 2 domain. Conversely, depletion of PXK using RNA interference decreased the rate of epidermal growth factor receptor internalization and degradation. Ubiquitination of epidermal growth factor receptor by the ligand stimulation was enhanced in PXK-expressing cells. These results indicate that PXK plays a critical role in epidermal growth factor receptor trafficking through modulating ligand-induced ubiquitination of the receptor.
Figures
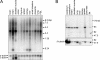

Similar articles
-
Expression pattern and subcellular localization of five splice isoforms of human PXK.Int J Mol Med. 2005 Oct;16(4):701-7. Int J Mol Med. 2005. PMID: 16142408
-
Structural basis for different phosphoinositide specificities of the PX domains of sorting nexins regulating G-protein signaling.J Biol Chem. 2014 Oct 10;289(41):28554-68. doi: 10.1074/jbc.M114.595959. Epub 2014 Aug 22. J Biol Chem. 2014. PMID: 25148684 Free PMC article.
-
The PtdIns3P-binding protein Phafin 2 mediates epidermal growth factor receptor degradation by promoting endosome fusion.Traffic. 2012 Nov;13(11):1547-63. doi: 10.1111/j.1600-0854.2012.01400.x. Epub 2012 Aug 8. Traffic. 2012. PMID: 22816767
-
The PX domain: a new phosphoinositide-binding module.J Cell Sci. 2002 Mar 15;115(Pt 6):1099-105. doi: 10.1242/jcs.115.6.1099. J Cell Sci. 2002. PMID: 11884510 Review.
-
SNX-PXA-RGS-PXC Subfamily of SNXs in the Regulation of Receptor-Mediated Signaling and Membrane Trafficking.Int J Mol Sci. 2021 Feb 26;22(5):2319. doi: 10.3390/ijms22052319. Int J Mol Sci. 2021. PMID: 33652569 Free PMC article. Review.
Cited by
-
Identification of novel molecular regulators of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in breast cancer cells by RNAi screening.Breast Cancer Res. 2014 Apr 17;16(2):R41. doi: 10.1186/bcr3645. Breast Cancer Res. 2014. PMID: 24745479 Free PMC article.
-
A systemic sclerosis and systemic lupus erythematosus pan-meta-GWAS reveals new shared susceptibility loci.Hum Mol Genet. 2013 Oct 1;22(19):4021-9. doi: 10.1093/hmg/ddt248. Epub 2013 Jun 4. Hum Mol Genet. 2013. PMID: 23740937 Free PMC article.
-
Regulation of the Phosphoinositide Code by Phosphorylation of Membrane Readers.Cells. 2021 May 14;10(5):1205. doi: 10.3390/cells10051205. Cells. 2021. PMID: 34069055 Free PMC article.
-
Proteomic profile and in silico analysis in metastatic melanoma with and without BRAF mutation.PLoS One. 2014 Dec 1;9(12):e112025. doi: 10.1371/journal.pone.0112025. eCollection 2014. PLoS One. 2014. PMID: 25437182 Free PMC article.
-
Distinct molecular targets of ProEGCG from EGCG and superior inhibition of angiogenesis signaling pathways for treatment of endometriosis.J Pharm Anal. 2024 Jan;14(1):100-114. doi: 10.1016/j.jpha.2023.09.005. Epub 2023 Sep 11. J Pharm Anal. 2024. PMID: 38352946 Free PMC article.
References
-
- Alwan, H. A., E. J. van Zoelen, and J. E. van Leeuwen. 2003. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J. Biol. Chem. 278:35781-35790. - PubMed
-
- Badour, K., M. K. McGavin, J. Zhang, S. Freeman, C. Vieira, D. Filipp, M. Julius, G. B. Mills, and K. A. Siminovitch. 2007. Interaction of the Wiskott-Aldrich syndrome protein with sorting nexin 9 is required for CD28 endocytosis and cosignaling in T cells. Proc. Natl. Acad. Sci. U. S. A. 104:1593-1598. - PMC - PubMed
-
- Boudeau, J., D. Miranda-Saavedra, G. J. Barton, and D. R. Alessi. 2006. Emerging roles of pseudokinases. Trends Cell Biol. 16:443-452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
