Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus
- PMID: 20086153
- PMCID: PMC2849374
- DOI: 10.1128/AAC.01625-09
Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus
Abstract
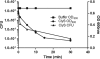
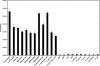
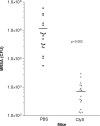
Staphylococcus aureus is the causative agent of several serious infectious diseases. The emergence of antibiotic-resistant S. aureus strains has resulted in significant treatment difficulties, intensifying the need for new antimicrobial agents. Toward this end, we have developed a novel chimeric bacteriophage (phage) lysin that is active against staphylococci, including methicillin-resistant S. aureus (MRSA). The chimeric lysin (called ClyS) was obtained by fusing the N-terminal catalytic domain of the S. aureus Twort phage lysin with the C-terminal cell wall-targeting domain from another S. aureus phage lysin (phiNM3), which displayed Staphylococcus-specific binding. ClyS was expressed in Escherichia coli, and the purified protein lysed MRSA, vancomycin-intermediate strains of S. aureus (VISA), and methicillin-sensitive (MSSA) strains of S. aureus in vitro. In a mouse nasal decolonization model, a 2-log reduction in the viability of MRSA cells was seen 1 h following a single treatment with ClyS. One intraperitoneal dose of ClyS also protected against death by MRSA in a mouse septicemia model. ClyS showed a typical pattern of synergistic interactions with both vancomycin and oxacillin in vitro. More importantly, ClyS and oxacillin at doses that were not protective individually protected synergistically against MRSA septic death in a mouse model. These results strongly support the development of ClyS as an attractive addition to the current treatment options of multidrug-resistant S. aureus infections and would allow for the reinstatement of antibiotics shelved because of mounting resistance.
Figures






References
-
- Aksoy, D. Y., and S. Unal.2008. New antimicrobial agents for the treatment of Gram-positive bacterial infections. Clin. Microbiol. Infect. 14:411-420. - PubMed
-
- Boyle-Vavra, S., and R. S. Daum.2007. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab. Investig. 87:3-9. - PubMed
-
- Brundage, J. F., and G. D. Shanks.2007. What really happened during the 1918 influenza pandemic? The importance of bacterial secondary infections. J. Infect. Dis. 196:1717-1718. - PubMed
-
- Centers for Disease Control and Prevention.2009. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May-August 2009. MMWR 58:1-4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

