Pharmacokinetics of chloroquine and monodesethylchloroquine in pregnancy
- PMID: 20086162
- PMCID: PMC2825967
- DOI: 10.1128/AAC.01269-09
Pharmacokinetics of chloroquine and monodesethylchloroquine in pregnancy
Abstract
In order to determine the pharmacokinetic disposition of chloroquine (CQ) and its active metabolite, desethylchloroquine (DECQ), when administered as intermittent presumptive treatment in pregnancy (IPTp) for malaria, 30 Papua New Guinean women in the second or third trimester of pregnancy and 30 age-matched nonpregnant women were administered three daily doses of 450 mg CQ (8.5 mg/kg of body weight/day) in addition to a single dose of sulfadoxine-pyrimethamine. For all women, blood was taken at baseline; at 1, 2, 4, 6, 12, 18, 24, 30, 48, and 72 h posttreatment; and at 7, 10, 14, 28, and 42 days posttreatment. Plasma was subsequently assayed for CQ and DECQ by high-performance liquid chromatography, and population pharmacokinetic modeling was performed. Pregnant subjects had significantly lower area under the plasma concentration-time curve for both CQ (35,750 versus 47,892 microg.h/liter, P < 0.001) and DECQ (23,073 versus 41,584 microg.h/liter, P < 0.001), reflecting significant differences in elimination half-lives and in volumes of distribution and clearances relative to bioavailability. Reduced plasma concentrations of both CQ and DECQ could compromise both curative efficacy and posttreatment prophylactic properties in pregnant patients. Higher IPTp CQ doses may be desirable but could increase the risk of adverse hemodynamic effects.
Figures
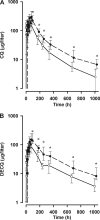

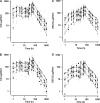
References
-
- Adjepon-Yamoah, K. K., D. Ofori-Adjei, N. M. Woolhouse, and B. Lindstrom. 1986. Whole-blood single-dose kinetics of chloroquine and desethylchloroquine in Africans. Ther. Drug Monit. 8:195-199. - PubMed
-
- Ajayi, F. O., L. A. Salako, and J. O. Kuye. 1989. Comparison of the partitioning in vitro of chloroquine and its desethyl metabolites between the erythrocytes and plasma of healthy subjects and those with falciparum malaria. Afr. J. Med. Med. Sci. 18:95-100. - PubMed
-
- Anderson, B. J., and N. H. Holford. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48:303-332. - PubMed
-
- Anderson, G. D. 2006. Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert Opin. Drug Metab. Toxicol. 2:947-960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

