Current multiple myeloma treatment strategies with novel agents: a European perspective
- PMID: 20086168
- PMCID: PMC3227886
- DOI: 10.1634/theoncologist.2009-0203
Current multiple myeloma treatment strategies with novel agents: a European perspective
Abstract
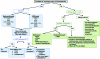
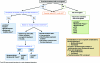
The treatment of multiple myeloma (MM) has undergone significant developments in recent years. The availability of the novel agents thalidomide, bortezomib, and lenalidomide has expanded treatment options and has improved the outcome of patients with MM. Following the introduction of these agents in the relapsed/refractory setting, they are also undergoing investigation in the initial treatment of MM. A number of phase III trials have demonstrated the efficacy of novel agent combinations in the transplant and nontransplant settings, and based on these results standard induction regimens are being challenged and replaced. In the transplant setting, a number of newer induction regimens are now available that have been shown to be superior to the vincristine, doxorubicin, and dexamethasone regimen. Similarly, in the front-line treatment of patients not eligible for transplantation, regimens incorporating novel agents have been found to be superior to the traditional melphalan plus prednisone regimen. Importantly, some of the novel agents appear to be active in patients with high-risk disease, such as adverse cytogenetic features, and certain comorbidities, such as renal impairment. This review presents an overview of the most recent data with these novel agents and summarizes European treatment practices incorporating the novel agents.
Conflict of interest statement
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
Figures
References
-
- Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. - PubMed
-
- van de Velde HJ, Liu X, Chen G, et al. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–1406. - PubMed
-
- Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114:3139–3146. - PubMed
-
- Lahuerta JJ, Mateos MV, Martínez-López J, et al. Influence of pre- and post-transplantation responses on outcome of patients with multiple myeloma: Sequential improvement of response and achievement of complete response are associated with longer survival. J Clin Oncol. 2008;26:5775–5782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

