Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction
- PMID: 20086198
- PMCID: PMC2853210
- DOI: 10.1152/ajpendo.00494.2009
Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction
Abstract
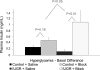
In this study, we examined chronic norepinephrine suppression of insulin secretion in sheep fetuses with placental insufficiency-induced intrauterine growth restriction (IUGR). Glucose-stimulated insulin secretion (GSIS) was measured with a square-wave hyperglycemic clamp in the presence or absence of adrenergic receptor antagonists phentolamine (alpha) and propranolol (beta). IUGR fetuses were hypoglycemic and hypoxemic and had lower GSIS responsiveness (P < or = 0.05) than control fetuses. IUGR fetuses also had elevated plasma norepinephrine (3,264 +/- 614 vs. 570 +/- 86 pg/ml; P < or = 0.05) and epinephrine (164 +/- 32 vs. 60 +/- 12 pg/ml; P < or = 0.05) concentrations. In control fetuses, adrenergic inhibition increased baseline plasma insulin concentrations (1.7-fold, P < or = 0.05), whereas during hyperglycemia insulin was not different. A greater (P < or = 0.05) response to adrenergic inhibition was found in IUGR fetuses, and the average plasma insulin concentrations increased 4.9-fold at baseline and 7.1-fold with hyperglycemia. Unlike controls, basal plasma glucose concentrations fell (P < or = 0.05) with adrenergic antagonists. GSIS responsiveness, measured by the change in insulin, was higher (8.9-fold, P < or = 0.05) in IUGR fetuses with adrenergic inhibition than controls (1.8-fold, not significant), showing that norepinephrine suppresses insulin secretion in IUGR fetuses. Strikingly, in IUGR fetuses, adrenergic inhibition resulted in a greater GSIS responsiveness, because beta-cell mass was 56% lower and the maximal stimulatory insulin response tended (P < 0.1) to be higher than controls. This persistent norepinephrine suppression appears to be partially explained by higher mRNA concentrations of adrenergic receptors alpha(1D), alpha(2A), and alpha(2B) in a cohort of fetuses that were naïve to the antagonists. Therefore, norepinephrine suppression of insulin secretion was maintained, in part, by upregulating adrenergic receptor expression, but the beta-cells also appeared to compensate with enhanced GSIS. These findings may begin to explain why IUGR infants have a propensity for increased glucose requirements if norepinephrine is suddenly decreased after birth.
Figures



Similar articles
-
Enhanced insulin secretion responsiveness and islet adrenergic desensitization after chronic norepinephrine suppression is discontinued in fetal sheep.Am J Physiol Endocrinol Metab. 2014 Jan 1;306(1):E58-64. doi: 10.1152/ajpendo.00517.2013. Epub 2013 Nov 19. Am J Physiol Endocrinol Metab. 2014. PMID: 24253046 Free PMC article.
-
Adrenal Demedullation and Oxygen Supplementation Independently Increase Glucose-Stimulated Insulin Concentrations in Fetal Sheep With Intrauterine Growth Restriction.Endocrinology. 2016 May;157(5):2104-15. doi: 10.1210/en.2015-1850. Epub 2016 Mar 3. Endocrinology. 2016. PMID: 26937714 Free PMC article.
-
Chronically elevated norepinephrine concentrations lower glucose uptake in fetal sheep.Am J Physiol Regul Integr Comp Physiol. 2020 Sep 1;319(3):R255-R263. doi: 10.1152/ajpregu.00365.2019. Epub 2020 Jul 15. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32667834 Free PMC article.
-
Fetal adaptations in insulin secretion result from high catecholamines during placental insufficiency.J Physiol. 2017 Aug 1;595(15):5103-5113. doi: 10.1113/JP273324. Epub 2017 May 26. J Physiol. 2017. PMID: 28194805 Free PMC article. Review.
-
Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism.J Pregnancy. 2012;2012:631038. doi: 10.1155/2012/631038. Epub 2012 Jul 31. J Pregnancy. 2012. PMID: 22900186 Free PMC article. Review.
Cited by
-
Impact of thermal stress on placental function and fetal physiology.Anim Reprod. 2018 Aug 3;15(Suppl 1):886-898. doi: 10.21451/1984-3143-AR2018-0056. eCollection 2018 Jul-Sep. Anim Reprod. 2018. PMID: 36249845 Free PMC article.
-
Sustained maternal inflammation during the early third-trimester yields intrauterine growth restriction, impaired skeletal muscle glucose metabolism, and diminished β-cell function in fetal sheep1,2.J Anim Sci. 2019 Dec 17;97(12):4822-4833. doi: 10.1093/jas/skz321. J Anim Sci. 2019. PMID: 31616931 Free PMC article.
-
Daily Injection of the β2 Adrenergic Agonist Clenbuterol Improved Muscle Glucose Metabolism, Glucose-Stimulated Insulin Secretion, and Hyperlipidemia in Juvenile Lambs Following Heat-Stress-Induced Intrauterine Growth Restriction.Metabolites. 2024 Mar 7;14(3):156. doi: 10.3390/metabo14030156. Metabolites. 2024. PMID: 38535316 Free PMC article.
-
Reducing Systemic Inflammation in IUGR-Born Neonatal Lambs via Daily Oral ω-3 PUFA Supplement Improved Skeletal Muscle Glucose Metabolism, Glucose-Stimulated Insulin Secretion, and Blood Pressure.Metabolites. 2025 May 22;15(6):346. doi: 10.3390/metabo15060346. Metabolites. 2025. PMID: 40559370 Free PMC article.
-
Pulsatile hyperglycemia increases insulin secretion but not pancreatic β-cell mass in intrauterine growth-restricted fetal sheep.J Dev Orig Health Dis. 2018 Oct;9(5):492-499. doi: 10.1017/S2040174418000417. Epub 2018 Jul 5. J Dev Orig Health Dis. 2018. PMID: 29973299 Free PMC article.
References
-
- Barker DJP. Mother, Babies and Health in Later Life Edinburgh: Churchill Livintstone, 1998
-
- Bassett JM, Hanson C. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. Am J Physiol Regul Integr Comp Physiol 274: R1536–R1545, 1998 - PubMed
-
- Bassett JM, Hanson C. Prevention of hypoinsulinemia modifies catecholamine effects in fetal sheep. Am J Physiol Regul Integr Comp Physiol 278: R1171–R1181, 2000 - PubMed
-
- Bassett JM, Symonds ME. Beta2-agonist ritodrine, unlike natural catecholamines, activates thermogenesis prematurely in fetal sheep. Am J Physiol Regul Integr Comp Physiol 275: R112–R119, 1998 - PubMed
-
- Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol 9: 17–29, 1987 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

