A battery of cell- and structure-specific markers for the adult porcine retina
- PMID: 20086234
- PMCID: PMC2842600
- DOI: 10.1369/jhc.2009.954933
A battery of cell- and structure-specific markers for the adult porcine retina
Abstract
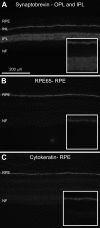
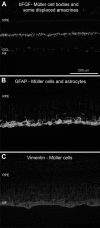
The pig is becoming an increasingly used non-primate model in experimental studies of human retinal diseases and disorders. The anatomy, size, and vasculature of the porcine eye and retina closely resemble their human counterparts, which allows for application of standard instrumentation and diagnostics used in the clinic. Despite many reports that demonstrate immunohistochemistry as a useful method for exploring neuropathological changes in the mammalian central nervous system, including the pig, the porcine retina has been sparsely described. Hence, to facilitate further immunohistochemical analysis of the porcine retina, we report on the successful use of a battery of antibodies for staining of paraformaldehyde-fixed cryosectioned retina. The following antibodies were evaluated for neuronal cells and structures: recoverin (cones and rods), Rho4D2 (rods), transducin-gamma (cones), ROM-1 (photoreceptor outer segments), calbindin (horizontal cells), PKC-alpha (bipolar cells), parvalbumin (amacrine and displaced amacrine cells), and NeuN (ganglion cells and displaced amacrines). For detecting synaptic connections in fiber layers, we used an antibody against synaptobrevin. For detecting retinal pigment epithelium, we studied antibodies against cytokeratin and RPE65, respectively. The glial cell markers used were bFGF (Müller cells and displaced amacrine cells), GFAP (Müller cells and astrocytes), and vimentin (Müller cells). Each staining effect was evaluated with regard to its specificity, sensitivity, and reproducibility in the identification of individual cells, specific cell structures, and fiber layers, respectively. The markers parvalbumin and ROM-1 were tested here for the first time for the porcine retina. All antibodies tested resulted in specific staining of high quality. In conclusion, all immunohistochemical protocols presented here will be applicable in fixed, cryosectioned pig retina.
Figures

Similar articles
-
N-methyl-N-nitrosourea-induced neuronal cell death in a large animal model of retinal degeneration in vitro.Exp Eye Res. 2016 Jul;148:55-64. doi: 10.1016/j.exer.2016.05.023. Epub 2016 May 26. Exp Eye Res. 2016. PMID: 27237409
-
Cell type differentiation dynamics in the developing porcine retina.Dev Neurosci. 2010 Mar;32(1):47-58. doi: 10.1159/000261704. Epub 2010 Feb 12. Dev Neurosci. 2010. PMID: 20150723
-
Photoreceptor and glial markers in human embryonic retina and in human embryonic retinal transplants to rat retina.Brain Res Dev Brain Res. 1994 Jul 15;80(1-2):81-95. doi: 10.1016/0165-3806(94)90092-2. Brain Res Dev Brain Res. 1994. PMID: 7955364
-
Immunohistochemical study of pig retinal development.Mol Vis. 2009 Sep 21;15:1915-28. Mol Vis. 2009. PMID: 19784390 Free PMC article.
-
The neurons of the ground squirrel retina as revealed by immunostains for calcium binding proteins and neurotransmitters.J Neurocytol. 2002 Sep-Nov;31(8-9):649-66. doi: 10.1023/a:1025791512555. J Neurocytol. 2002. PMID: 14501205
Cited by
-
Tools and Biomarkers for the Study of Retinal Ganglion Cell Degeneration.Int J Mol Sci. 2022 Apr 13;23(8):4287. doi: 10.3390/ijms23084287. Int J Mol Sci. 2022. PMID: 35457104 Free PMC article. Review.
-
Progression of Pro23His Retinopathy in a Miniature Swine Model of Retinitis Pigmentosa.Transl Vis Sci Technol. 2017 Mar 15;6(2):4. doi: 10.1167/tvst.6.2.4. eCollection 2017 Mar. Transl Vis Sci Technol. 2017. PMID: 28316877 Free PMC article.
-
Potential neuroprotective effect of stem cells from apical papilla derived extracellular vesicles enriched by lab-on-chip approach during retinal degeneration.Cell Mol Life Sci. 2022 Jun 7;79(7):350. doi: 10.1007/s00018-022-04375-2. Cell Mol Life Sci. 2022. PMID: 35672609 Free PMC article.
-
Wound Healing in a Porcine Model of Retinal Holes.Invest Ophthalmol Vis Sci. 2024 Aug 1;65(10):35. doi: 10.1167/iovs.65.10.35. Invest Ophthalmol Vis Sci. 2024. PMID: 39186262 Free PMC article.
-
Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice.Mol Vis. 2012;18:1411-20. Epub 2012 Jun 1. Mol Vis. 2012. PMID: 22690119 Free PMC article.
References
-
- Ahn M, Moon C, Jung C, Kim H, Jin JK, Shin T (2009) Immunohistochemical localization of protein kinase C-alpha in the retina of pigs during postnatal development. Neurosci Lett 455:93–96 - PubMed
-
- Beauchemin ML (1974) The fine structure of the pig's retina. Graefes Arch Klin Exp Ophthalmol 190:27–45 - PubMed
-
- Bignami A, Dahl D (1979) The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res 28:63–69 - PubMed
-
- Chader GJ (2002) Animal models in research on retinal degenerations: past progress and future hope. Vision Res 42:393–399 - PubMed
-
- Chandler MJ, Smith PJ, Samuelson DA, MacKay EO (1999) Photoreceptor density of the domestic pig retina. Vet Ophthalmol 2:179–184 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

