Regulation of primary alloantibody response through antecedent exposure to a microbial T-cell epitope
- PMID: 20086249
- PMCID: PMC2869558
- DOI: 10.1182/blood-2009-08-238568
Regulation of primary alloantibody response through antecedent exposure to a microbial T-cell epitope
Abstract
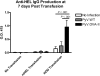
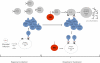
Humoral alloimmunization to red blood cell (RBC) antigens is a clinically significant problem that can lead to transfusion reactions and difficulty in locating future compatible blood for transfusion. However, factors regulating responder/nonresponder status are only partially understood. Herein, we identify a series of microbes with 100% identity in 8- to 9-amino acid peptides containing the variant amino acids in Kell, Kidd, and Duffy antigens. To test the hypothesis that infection with such a microbe could predispose to RBC alloimmunization, a mouse model was developed using murine polyoma virus expressing a defined CD4(+) T-cell epitope ovalbumin(323-339) ((OVA)(323-339)) and subsequent transfusion with RBCs expressing a B-cell epitope (hen egg lysozyme [HEL]) fused to (OVA)(323-339). Whereas infection alone induced no detectable anti-HEL, subsequent RBC transfusion induced 100- to 1000-fold more anti-HEL in mice that had been previously infected compared with control mice. This effect did not occur with wild-type polyoma virus or RBCs expressing HEL alone. Together, these data indicate that prior exposure to a pathogen with small peptide homology to RBC antigens can lead to an enhanced primary alloantibody response. As such priming is not detectable by current clinical tests, it is unknown to what extent this occurs in human alloimmunization.
Figures




References
-
- Moise KJ. Fetal anemia due to non-rhesus-D red-cell alloimmunization. Semin Fetal Neonatal Med. 2008;13(4):207–214. - PubMed
-
- Hillyer CD, Silberstein LE, Ness PM, Anderson KC, Roback JD. Blood Banking and Transfusion Medicine: Basic Principles and Practice. 2nd ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2007.
-
- Heddle NM, Soutar RL, O'Hoski PL, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91(4):1000–1005. - PubMed
-
- Yazer MH, Triulzi DJ. Detection of anti-D in D- recipients transfused with D+ red blood cells. Transfusion. 2007;47(12):2197–2201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

