Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients
- PMID: 20087132
- PMCID: PMC2939497
- DOI: 10.1227/01.NEU.0000360379.95800.2F
Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients
Abstract
Objective: This work evaluated the clinical feasibility of transcranial magnetic resonance imaging-guided focused ultrasound surgery.
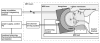
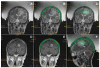
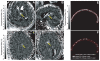
Methods: Transcranial magnetic resonance imaging-guided focused ultrasound surgery offers a potential noninvasive alternative to surgical resection. The method combines a hemispherical phased-array transducer and patient-specific treatment planning based on acoustic models with feedback control based on magnetic resonance temperature imaging to overcome the effects of the cranium and allow for controlled and precise thermal ablation in the brain. In initial trials in 3 glioblastoma patients, multiple focused ultrasound exposures were applied up to the maximum acoustic power available. Offline analysis of the magnetic resonance temperature images evaluated the temperature changes at the focus and brain surface.
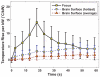
Results: We found that it was possible to focus an ultrasound beam transcranially into the brain and to visualize the heating with magnetic resonance temperature imaging. Although we were limited by the device power available at the time and thus seemed to not achieve thermal coagulation, extrapolation of the temperature measurements at the focus and on the brain surface suggests that thermal ablation will be possible with this device without overheating the brain surface, with some possible limitation on the treatment envelope.
Conclusion: Although significant hurdles remain, these findings are a major step forward in producing a completely noninvasive alternative to surgical resection for brain disorders.
Figures


References
-
- Astrom KE, Belle, Ballantine HT, Heidensleben E. An experimental neuropathological study of the effects of high-frequency focused ultrasound on the brain of the cat. J Neuropathol Exp Neurol. 1961;20:484–520. - PubMed
-
- Aubry JF, Tanter M, Pernot M, Thomas JL, Fink M. Experimental demonstration of noninvasive transskull adaptive focusing based on prior computed tomography scans. J Acoust Soc Am. 2003;113:84–93. - PubMed
-
- Barnard JW, Fry WJ, Fry FJ, Krumins RF. Effects of high intensity ultrasound on the central nervous system of the cat. J Comp Neurol. 1955;103:459–484. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

