Guanine analogues enhance antisense oligonucleotide-induced exon skipping in dystrophin gene in vitro and in vivo
- PMID: 20087314
- PMCID: PMC2862535
- DOI: 10.1038/mt.2009.320
Guanine analogues enhance antisense oligonucleotide-induced exon skipping in dystrophin gene in vitro and in vivo
Abstract
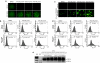
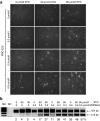
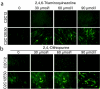
Exon skipping has demonstrated great potential for treating Duchenne muscular dystrophy (DMD) and other diseases. We have developed a drug-screening system using C2C12 myoblasts expressing a reporter green fluorescent phosphate (GFP), with its reading frame disrupted by the insertion of a targeted dystrophin exon. A library of 2,000 compounds (Spectrum collection; Microsource Discovery System) was screened to identify drugs capable of skipping targeted dystrophin exons or enhancing the exon-skipping effect by specific antisense oligomers. The 6-thioguanine (6TG) was effective for inducing skipping of both human dystrophin exon 50 (hDysE50) and mouse dystrophin exon 23 (mDysE23) in the cell culture systems and increased exon skipping efficiency (more than threefolds) when used in combination with phosphorodiamidate morpholino oligomers (PMO) in both myoblasts and myotubes. Guanine and its analogues were unable to induce detectable skipping of exon 23 when used alone but enhanced PMO-induced exon skipping significantly (approximately two times) in the muscles of dystrophic mdx mouse in vivo. Our results demonstrate that small-molecule compounds could enhance specific exon skipping synergistically with antisense oligomers for experimental therapy to human diseases.
Figures


Similar articles
-
In Vitro Multiexon Skipping by Antisense PMOs in Dystrophic Dog and Exon 7-Deleted DMD Patient.Methods Mol Biol. 2018;1828:151-163. doi: 10.1007/978-1-4939-8651-4_9. Methods Mol Biol. 2018. PMID: 30171540 Free PMC article.
-
Targeted skipping of human dystrophin exons in transgenic mouse model systemically for antisense drug development.PLoS One. 2011;6(5):e19906. doi: 10.1371/journal.pone.0019906. Epub 2011 May 17. PLoS One. 2011. PMID: 21611204 Free PMC article.
-
In Vitro Multiexon Skipping by Antisense PMOs in Dystrophic Dog and Exon 7-Deleted DMD Patient.Methods Mol Biol. 2025;2964:143-155. doi: 10.1007/978-1-0716-4730-1_9. Methods Mol Biol. 2025. PMID: 40720016
-
Skipping multiple exons of dystrophin transcripts using cocktail antisense oligonucleotides.Nucleic Acid Ther. 2014 Feb;24(1):57-68. doi: 10.1089/nat.2013.0451. Epub 2013 Dec 31. Nucleic Acid Ther. 2014. PMID: 24380394 Review.
-
Rescue of dystrophin mRNA of Duchenne muscular dystrophy by inducing exon skipping.Acta Myol. 2005 Oct;24(2):110-4. Acta Myol. 2005. PMID: 16550927 Review.
Cited by
-
Aminoglycoside Enhances the Delivery of Antisense Morpholino Oligonucleotides In Vitro and in mdx Mice.Mol Ther Nucleic Acids. 2019 Jun 7;16:663-674. doi: 10.1016/j.omtn.2019.04.023. Epub 2019 May 2. Mol Ther Nucleic Acids. 2019. PMID: 31121478 Free PMC article.
-
Ongoing therapeutic trials and outcome measures for Duchenne muscular dystrophy.Cell Mol Life Sci. 2013 Dec;70(23):4585-602. doi: 10.1007/s00018-013-1396-z. Epub 2013 Jun 18. Cell Mol Life Sci. 2013. PMID: 23775131 Free PMC article. Review.
-
Evaluation of Tris[2-(acryloyloxy)ethyl]isocyanurate cross-linked polyethylenimine as antisense morpholino oligomer delivery vehicle in cell culture and dystrophic mdx mice.Hum Gene Ther. 2014 May;25(5):419-27. doi: 10.1089/hum.2013.156. Epub 2014 Apr 25. Hum Gene Ther. 2014. PMID: 24405395 Free PMC article.
-
Triazine-cored polymeric vectors for antisense oligonucleotide delivery in vitro and in vivo.J Nanobiotechnology. 2020 Feb 18;18(1):34. doi: 10.1186/s12951-020-0586-8. J Nanobiotechnology. 2020. PMID: 32070342 Free PMC article.
-
Saponins enhance exon skipping of 2'-O-methyl phosphorothioate oligonucleotide in vitro and in vivo.Drug Des Devel Ther. 2018 Oct 31;12:3705-3715. doi: 10.2147/DDDT.S179008. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30464402 Free PMC article.
References
-
- Hoffman EP., and , Kunkel LM. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989;2:1019–1029. - PubMed
-
- Koenig M, Monaco AP., and , Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. - PubMed
-
- Hoffman EP, Brown RH., Jr, and , Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Michalak M., and , Opas M. Functions of dystrophin and dystrophin associated proteins. Curr Opin Neurol. 1997;10:436–442. - PubMed
-
- Straub V., and , Campbell KP. Muscular dystrophies and the dystrophin-glycoprotein complex. Curr Opin Neurol. 1997;10:168–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

