Recent advances in lentiviral vector development and applications
- PMID: 20087315
- PMCID: PMC2839421
- DOI: 10.1038/mt.2009.319
Recent advances in lentiviral vector development and applications
Erratum in
-
Corrigendum to "Recent Advances in Lentiviral Vector Development and Applications".Mol Ther. 2010 May;18(5):1055. doi: 10.1038/mt.2010.15. Epub 2016 Dec 6. Mol Ther. 2010. PMID: 28178555 Free PMC article. No abstract available.
Abstract
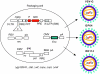
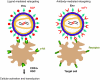
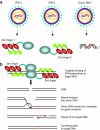
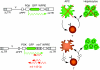
Lentiviral vectors (LVs) have emerged as potent and versatile vectors for ex vivo or in vivo gene transfer into dividing and nondividing cells. Robust phenotypic correction of diseases in mouse models has been achieved paving the way toward the first clinical trials. LVs can deliver genes ex vivo into bona fide stem cells, particularly hematopoietic stem cells, allowing for stable transgene expression upon hematopoietic reconstitution. They are also useful to generate induced pluripotent stem cells. LVs can be pseudotyped with distinct viral envelopes that influence vector tropism and transduction efficiency. Targetable LVs can be generated by incorporating specific ligands or antibodies into the vector envelope. Immune responses toward the transgene products and transduced cells can be repressed using microRNA-regulated vectors. Though there are safety concerns regarding insertional mutagenesis, their integration profile seems more favorable than that of gamma-retroviral vectors (gamma-RVs). Moreover, it is possible to minimize this risk by modifying the vector design or by employing integration-deficient LVs. In conjunction with zinc-finger nuclease technology, LVs allow for site-specific gene correction or addition in predefined chromosomal loci. These recent advances underscore the improved safety and efficacy of LVs with important implications for clinical trials.
Figures
References
-
- May C, Rivella S, Callegari J, Heller G, Gaensler KM, Luzzatto L, et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature. 2000;406:82–86. - PubMed
-
- Sinn PL, Sauter SL., and , McCray PB., Jr Gene therapy progress and prospects: development of improved lentiviral and retroviral vectors–design, biosafety, and production. Gene Ther. 2005;12:1089–1098. - PubMed
-
- Trono D. Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Ther. 2000;7:20–23. - PubMed
-
- Vigna E., and , Naldini L. Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy. J Gene Med. 2000;2:308–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

